Method for preparing 2-amino-2-methyl-1-propionic ester
A technology of propionate and methyl, applied in the field of preparation of 2-amino-2-methyl-1-propionate, can solve the problems of low conversion rate, recrystallization, long reaction holding time, etc., and achieves a wide range of sources. , the route cost is low, and the reaction steps are simple to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
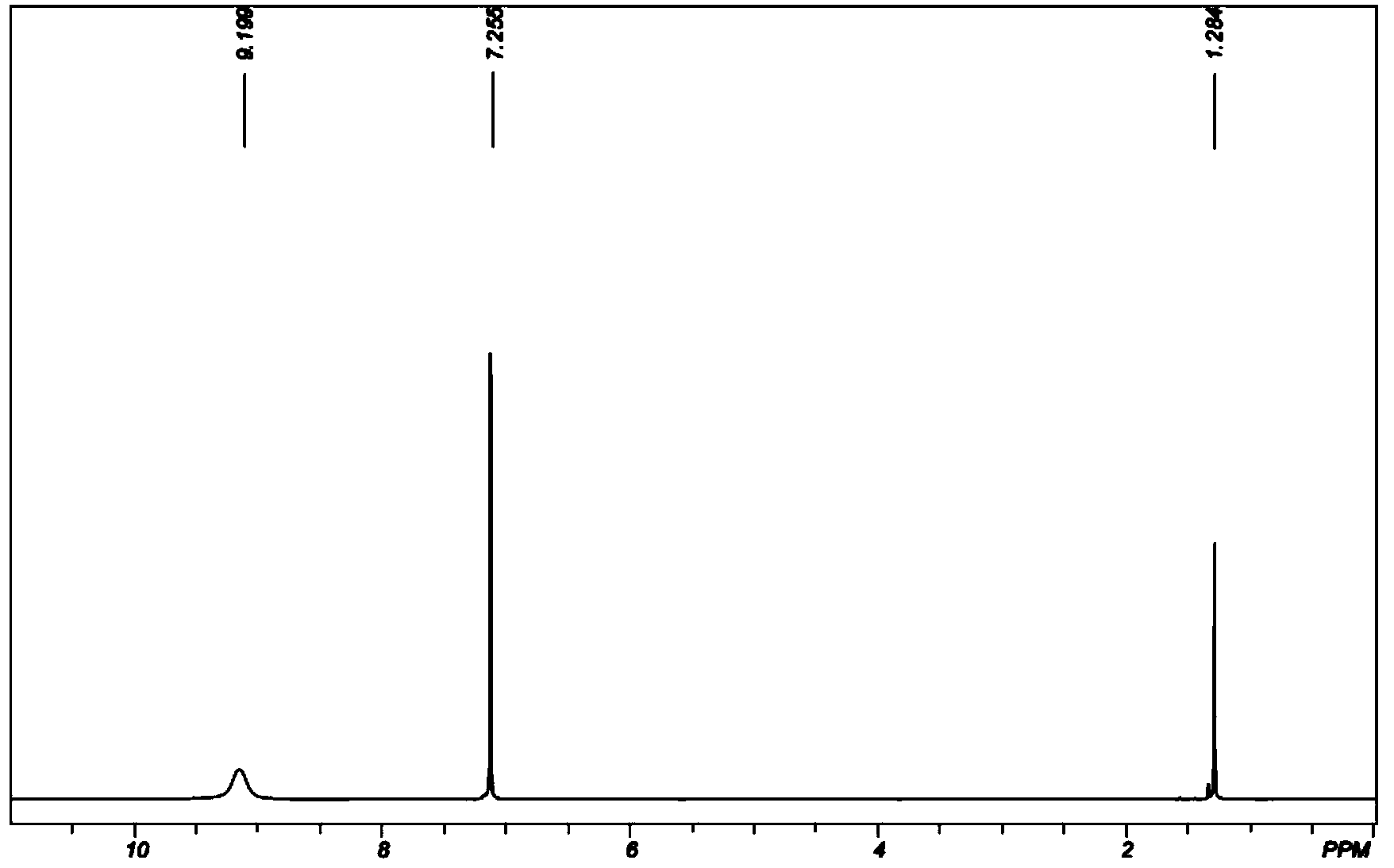
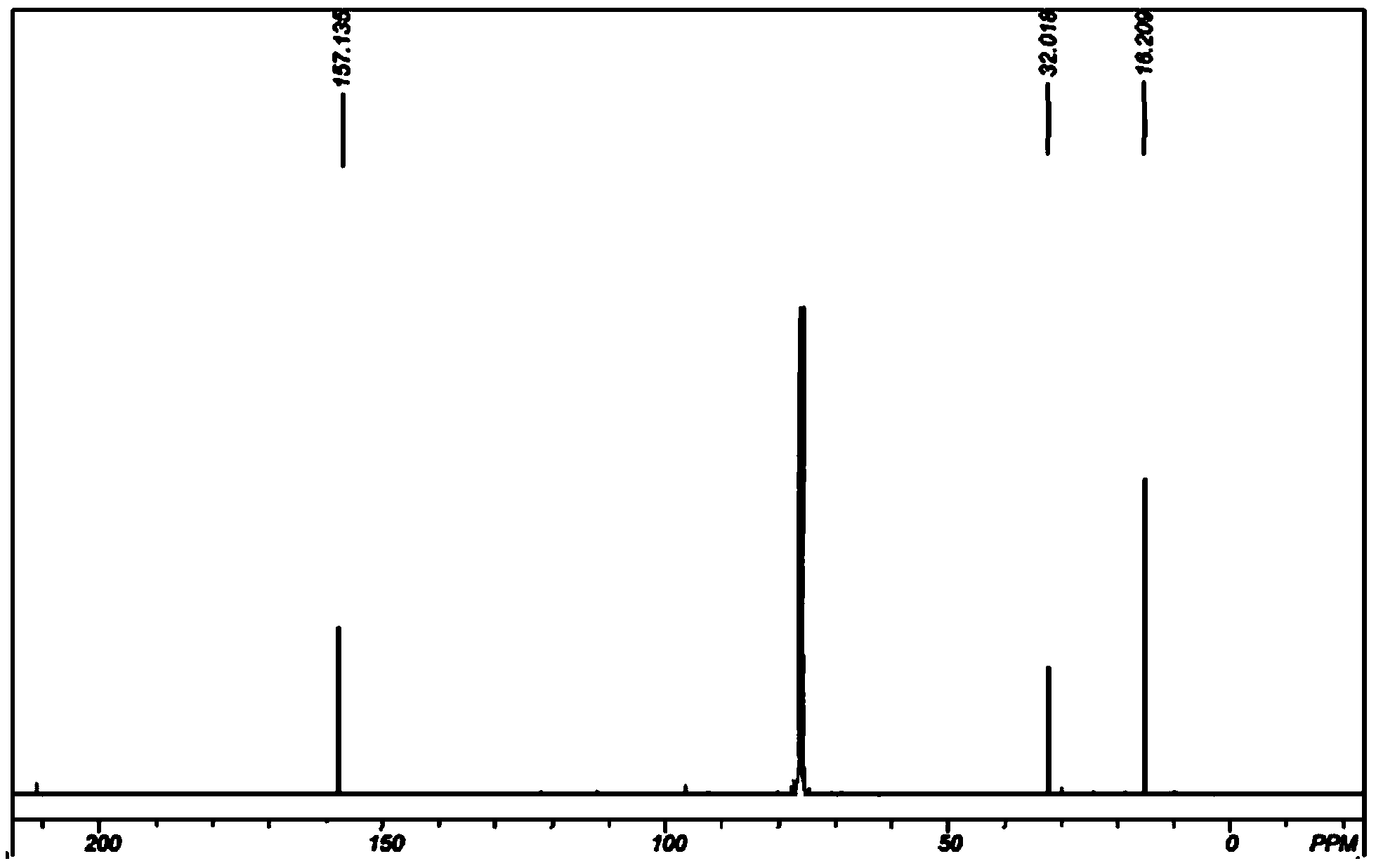
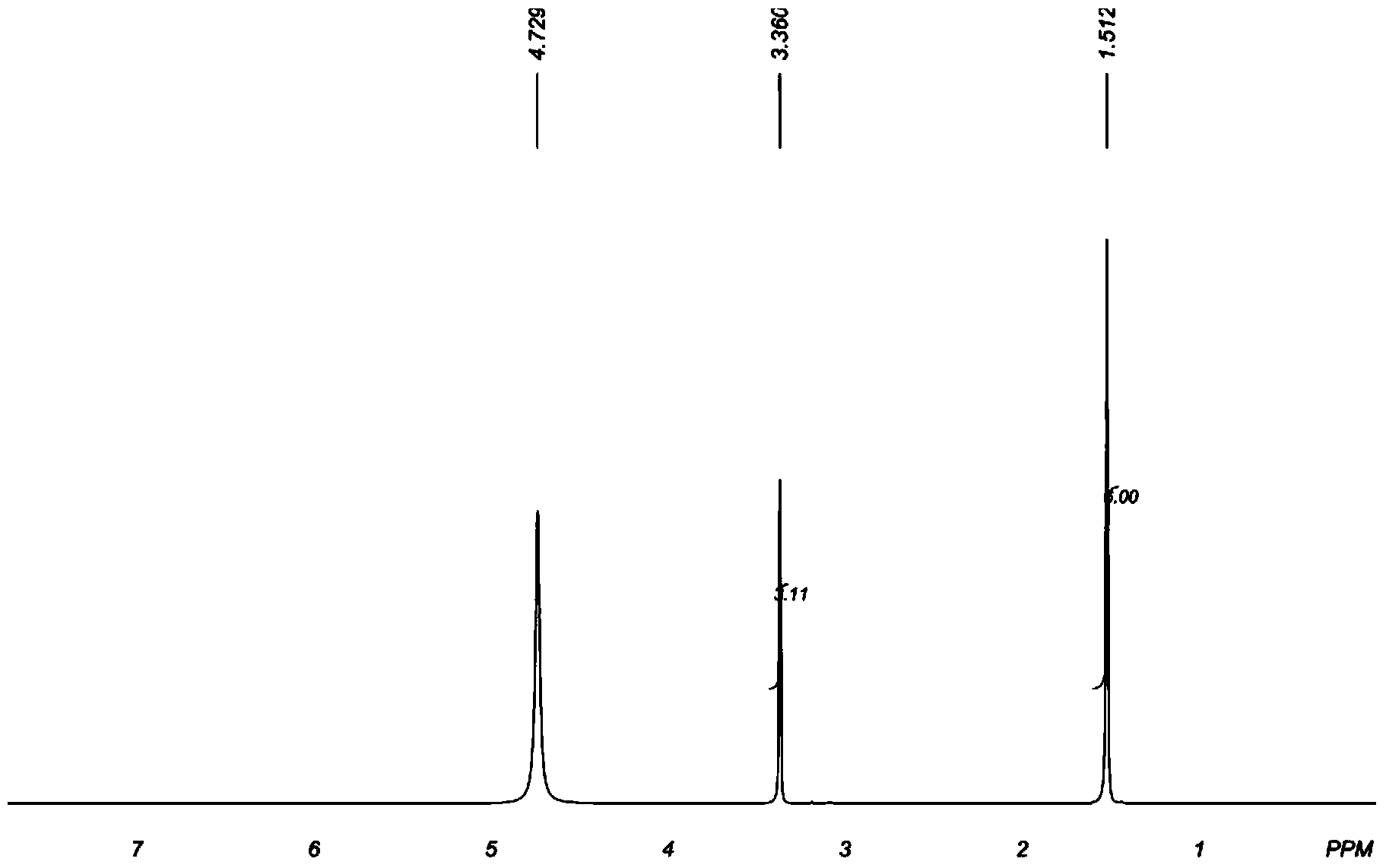
Image
Examples
Embodiment 1
[0049] (1). Dimerization of dimethyl ketene: Weigh 70.04g (1.00mol) dimethyl ketene and dissolve it in 200g ethyl acetate, put the ethyl acetate solution dissolved in dimethyl ketene in 500ml In a three-necked flask, raise the temperature to 70° C., and keep it warm for 2 hours to obtain a dimerization reaction solution. Ethyl acetate was distilled off from the reaction solution at 0.02 MPa and 50° C. to obtain 69.88 g of solid.
[0050] (2). Preparation of 2,2,4,4-tetramethyl-1,3-cyclobutanedione oxime: 69.88g of the solid obtained in step (1) (based on the complete reaction of dimethyl ketene, 0.50mol ) and 70.19g (1.01mol) of hydroxylamine hydrochloride were placed in a 500ml three-necked flask containing 160g (5.00mol) of methanol, after the temperature was raised to 20°C, ammonia was slowly introduced into the reaction system under stirring, and the reaction temperature was maintained at 20°C. The pH of the system was 8-12. After continuing to pass through for 4 hours, s...
Embodiment 2
[0061] (1).Dimerization of dimethylketene: Weigh 210.12g (1.50mol) dimerization product 2,2,4,4-tetramethyl-1,3-cyclobutanedione of dimethylketene Add it into a 500ml three-necked flask, raise the temperature to 120°C, add 70.04g (1.00mol) of dimethyl ketene dropwise under normal pressure, after the addition is complete, continue to raise the temperature to 140°C, and keep it warm for 1.5h to obtain dimethicone Polymerization solution. After cooling to room temperature, 277.32 g of solid were obtained.
[0062] (2). Preparation of 2,2,4,4-tetramethyl-1,3-cyclobutanedione oxime: 69.33 g of the solid obtained in step (1) (based on the complete reaction of dimethyl ketene, 0.50 mol ) and 82.89g (1.01mol) of hydroxylamine sulfate were placed in a 1000ml three-necked flask containing 480g (15.00mol) of methanol, after the temperature was raised to 40°C, solid sodium carbonate was slowly added to the reaction system under stirring, and the reaction temperature was kept at 40°C. Th...
Embodiment 3
[0066] (1). Dimerization of dimethyl ketene: Weigh 70.04g (1.00mol) dimethyl ketene and dissolve it in 140g ethyl acetate, and place the ethyl acetate solution dissolved in dimethyl ketene in 500ml In a three-necked flask, raise the temperature to 110° C., and keep it warm for 3 hours to obtain a dimerization reaction solution. The reaction solution was evaporated to dry ethyl acetate at 0.02 MPa and 50° C. to obtain 69.45 g of solid.
[0067] (2). Preparation of 2,2,4,4-tetramethyl-1,3-cyclobutanedione oxime: 69.45 g of the solid obtained in step (1) (based on the complete reaction of dimethyl ketene, 0.50 mol ) and 68.98g (1.05mol) of hydroxylamine phosphate were placed in a 500ml three-neck flask containing 160g (5.00mol) of methanol, and after the temperature was raised to 40°C, ammonia gas was slowly introduced into the reaction system under stirring, and the reaction temperature was kept at 40°C. The pH of the system is 8-12. After continuous feeding for 2 hours, the fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com