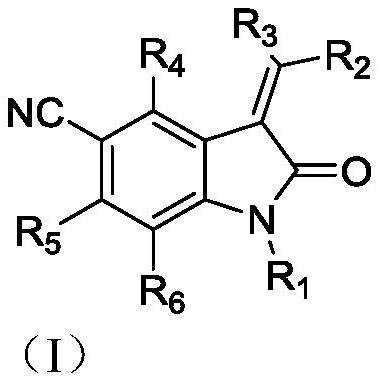
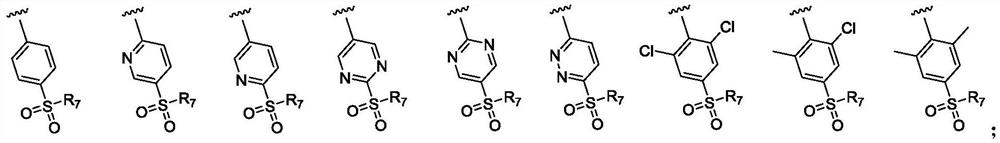
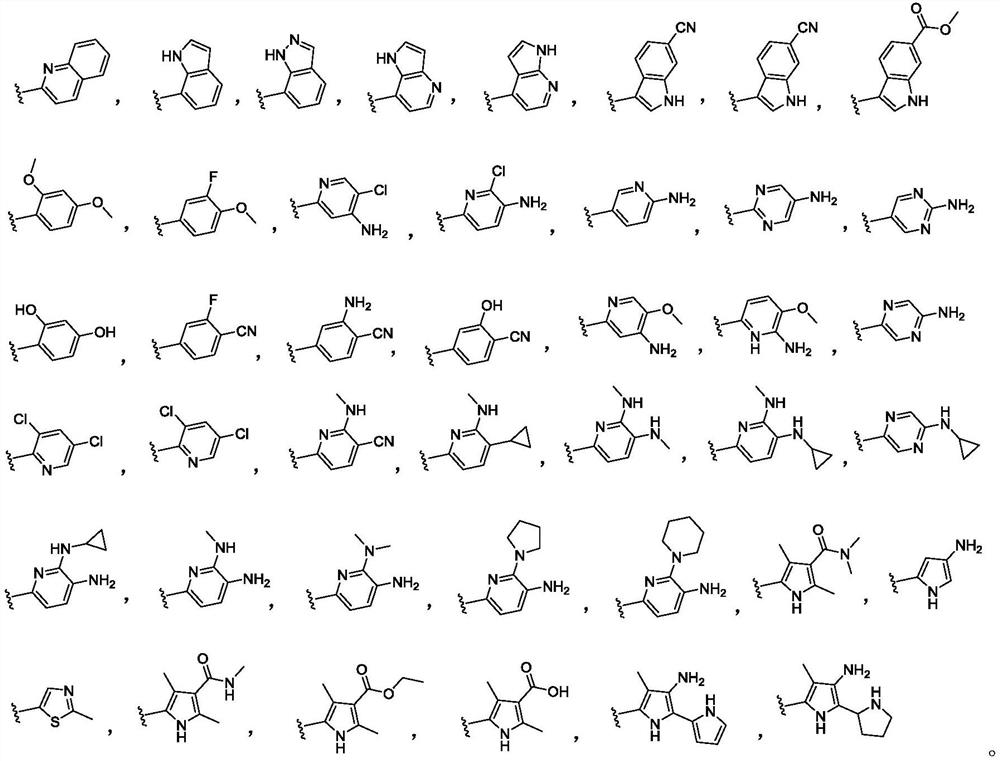
Indole-2-ketone compound as well as preparation method and application thereof
A technology of ketone compounds and compounds, which is applied in the field of indole-2-one compounds and their preparation, can solve the problems of unavailable drugs and achieve important practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 (Z)-3-((4-amino-1H-pyrrol-2-yl)methylene)-6-methyl-1-(4-(methylsulfonyl)phenyl)-2- Preparation of Oxoindoline-5-carbonitrile (Compound I-1)
[0118]
[0119] Adopt the following synthetic synthesis route:
[0120]
[0121] Step 1: Synthesis of (Z)-6-methyl-1-(4-(methylsulfonyl)phenyl)-3-((4-nitro-1H-pyrrol-2-yl)methylene)- 2-Oxoindoline-5-carbonitrile
[0122] Intermediate A-1 (163 mg, 0.5 mmol) and 4-nitro-1H-pyrrole-2-carbaldehyde (70 mg, 0.5 mmol) were dissolved in 20 mL of absolute ethanol with stirring, and two drops of piperidine were added dropwise, gradually turning yellow A solid precipitated out, and the reaction was continued overnight at room temperature. TLC detected that all the raw materials disappeared, filtered, the filter cake was rinsed with a small amount of cold ethanol, and dried to obtain 116 mg of a yellow solid, yield: 52%. TL C: Rf0.5 (petroleum ether: ethyl acetate = 1:1). ESI-MS: [M+1]=449.
[0123] Step 2: Synthesis of ...
Embodiment 2
[0126] Example 2 (Z)-3-((5-amino-6-chloropyridin-2-yl)methylene)-6-methyl-1-(4-(methylsulfonyl)phenyl)-2 - Preparation of oxoindoline-5-carbonitrile (compound 1-2)
[0127]
[0128] Adopt the following synthetic route:
[0129]
[0130] Step 1: Synthesis of (Z)-3-((6-chloro-5-nitropyridin-2-yl)methylene)-6-methyl-1-(4-(methylsulfonyl)phenyl) -2-Oxoindoline-5-carbonitrile
[0131] Intermediate A-1 (163 mg, 0.5 mmol) and 6-chloro-5-nitro-2-formylpyridine (92 mg, 0.5 mmol) were dissolved in 20 mL of absolute ethanol with stirring, and two drops of triethylamine were added dropwise, Raise the temperature to 80°C to react overnight. It was detected by TLC that all the raw materials disappeared, the reaction solution was concentrated, and purified by silica gel column chromatography (DCM:MeOH=30:1) to obtain 160 mg of yellow solid, yield: 65%. ESI-MS: [M+1]=495.
[0132] Step 2: Synthesis of (Z)-3-((5-amino-6-chloropyridin-2-yl)methylene)-6-methyl-1-(4-(methylsulfonyl)phe...
Embodiment 3
[0135] Example 3 (Z)-3-((5-amino-6-(piperidin-1-yl)pyridin-2-yl)methylene)-1-(4-(methylsulfonyl)phenyl) - Preparation of 2-oxoindoline-5-carbonitrile (compound 1-3)
[0136]
[0137] Adopt the following synthetic route:
[0138]
[0139] Step 1: Synthesis of (Z)-1-(4-(methylsulfonyl)phenyl)-3-((5-nitro-6-(piperidin-1-yl)pyridin-2-yl)methylene Base) -2-oxoindoline-5-carbonitrile
[0140] Intermediate A-2 (156mg, 0.5mmol) and 6-chloro-5-nitro-2-formylpyridine (93mg, 0.5mmol) were dissolved in 20mL absolute ethanol with stirring, and piperidine (64mg, 0.75 mmol), the temperature was raised to 80°C for overnight reaction. It was detected by TLC that all the raw materials disappeared, the reaction solution was concentrated, and purified by silica gel column chromatography (DCM:MeOH=30:1) to obtain 122 mg of a yellow solid, yield: 46%. ESI-MS: [M+1]=530;
[0141] Step 2: Synthesis of (Z)-3-((5-amino-6-(piperidin-1-yl)pyridin-2-yl)methylene)-1-(4-(methylsulfonyl)phenyl )-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com