Keratinocyte growth factor active polypeptide and application thereof
A technology for growth factor activity and keratinocytes, which is applied in the field of keratinocyte growth factor active polypeptides, and can solve problems such as no active polypeptide gels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
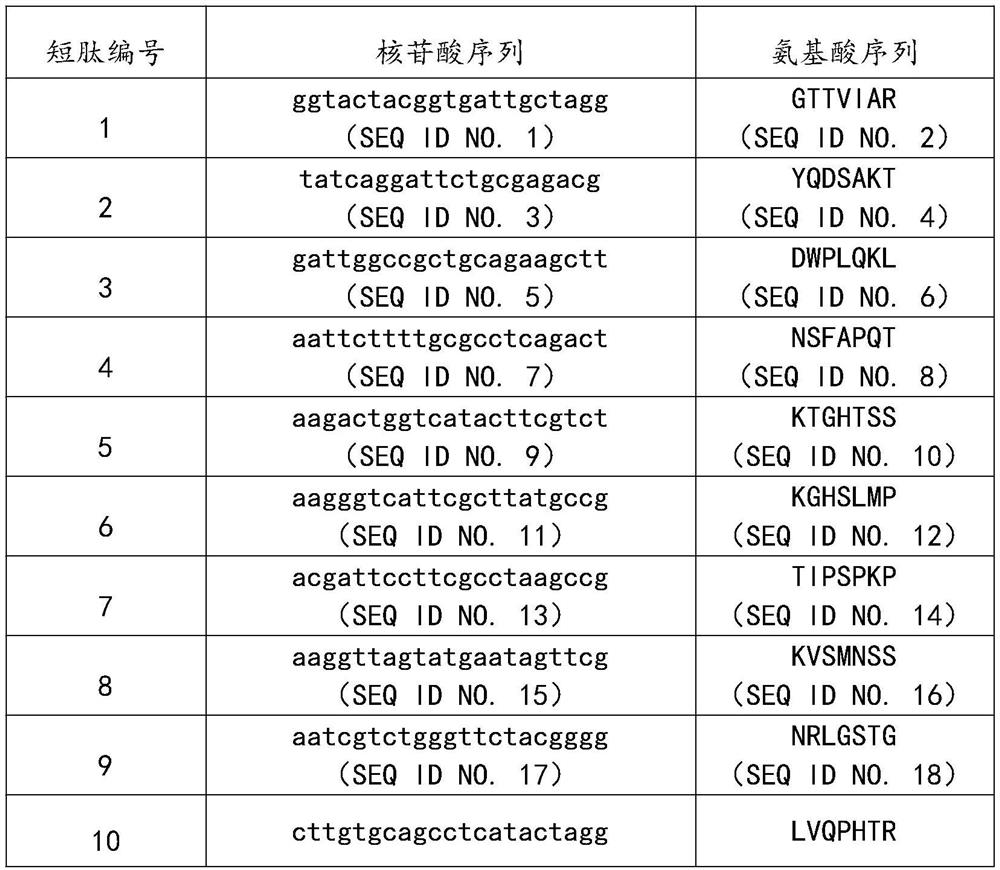
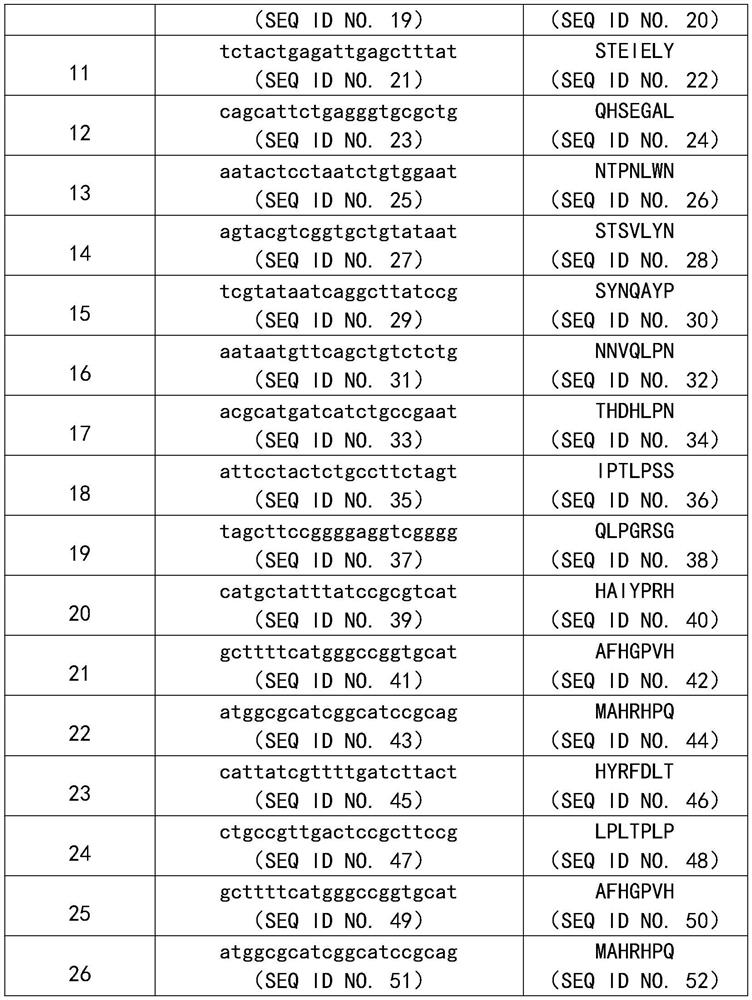
[0032] Embodiment 1: Screening of KGF active polypeptide sequence
[0033] Coat the 96-well plate with human KGF monoclonal antibody, add phage random heptapeptide library (purchased from NEB Company, USA) and incubate overnight at 4°C, wash the plate 10 times with TBST, and wash the plate with elution buffer (0.2mol / L Glycine-HCl , pH value of 2.2, 0.1% BSA) to elute the bound phage, neutralization buffer (1mol / L Tris-HCl, pH value of 9.1) quickly neutralizes the eluate.
[0034] E.coli ER2738 host bacteria were used to amplify the eluate for the next round of screening. In the same way, three rounds of screening were performed on the eluate from each round. Titer determination was carried out on the eluate of the fourth round, phage coeruleus was randomly selected, amplified, and adjusted to 2.0×10 13 pfu / mL.
[0035] The ELISA method and the human KGF immunoassay kit (purchased from R&D Company, USA) were used to detect the binding ability of the monoclonal phage accordi...
Embodiment 2
[0047] Embodiment 2: the synthesis of KGF active polypeptide
[0048] The KGF polypeptide sequence obtained by screening in Example 1 was sent to Wuhan Minghao Biotechnology Co., Ltd. for the synthesis of KGF active short peptide, purified to 98% purity, and then the C-terminus of the polypeptide was amidated and blocked to enhance the stability of the active polypeptide ; Rhodamine laser dye labeling of the N-terminus of the polypeptide is used to identify the affinity of the active polypeptide to epidermal cells and to detect whether it can be combined with epidermal cells. Prepared into freeze-dried powder and stored at -20°C.
Embodiment 3
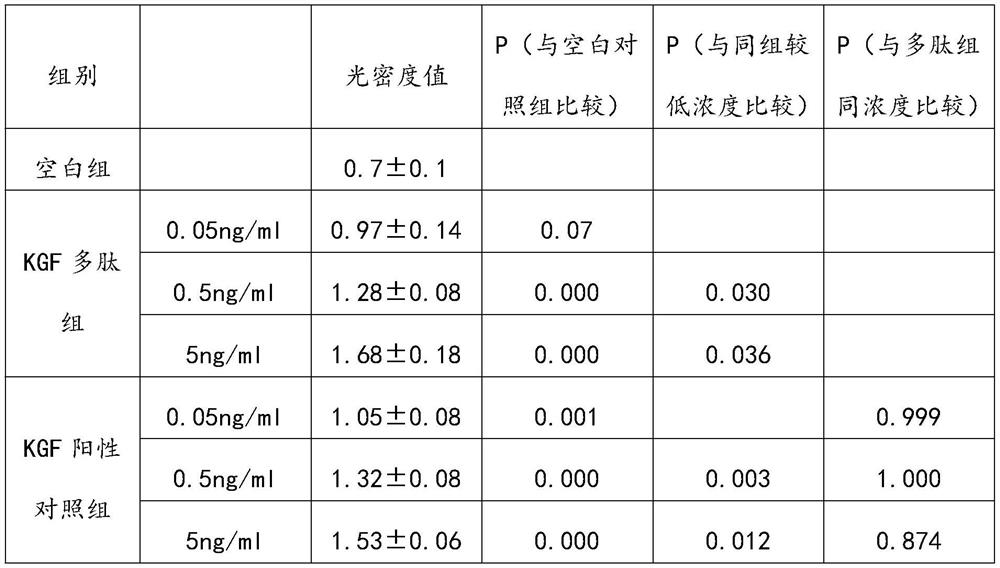
[0049] Embodiment 3: In vivo and in vitro experiments of KGF active polypeptide
[0050] The cultured epidermal cells were intervened with KGF active peptides, because the KGF active peptides were labeled with rhodamine laser dye, and the results of immunofluorescence detection showed that the KGF active peptides could combine with the specific receptor KGFR on the epidermal cells.
[0051] The KGF active polypeptide was further co-cultured with human epidermal cells for in vitro experiments, and the effect of the KGF active polypeptide on the proliferation of epidermal cells was detected by the CCK-8 method. The results showed that the KGF active polypeptide could promote the proliferation of epidermal cells in a concentration-dependent manner. RT-PCR and Western-blot were used to detect the expression level of the specific receptor KGFR on the epidermal cells, and the results showed that the KGF active polypeptide could promote the expression of KGFR on the epidermal cells. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com