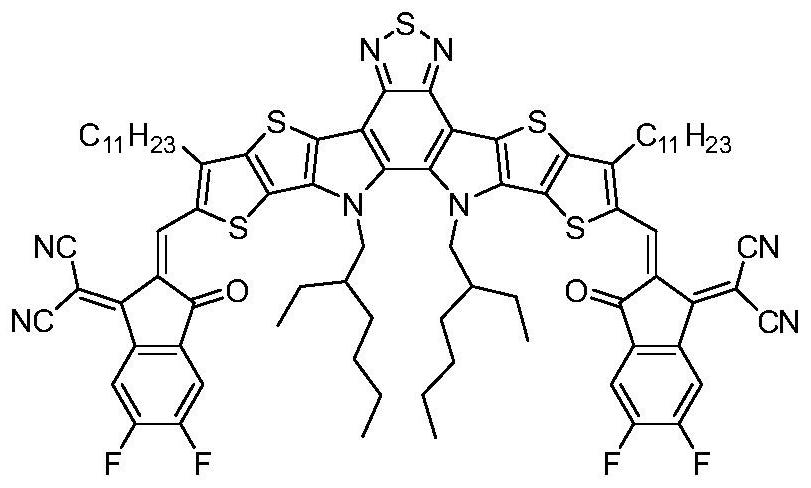
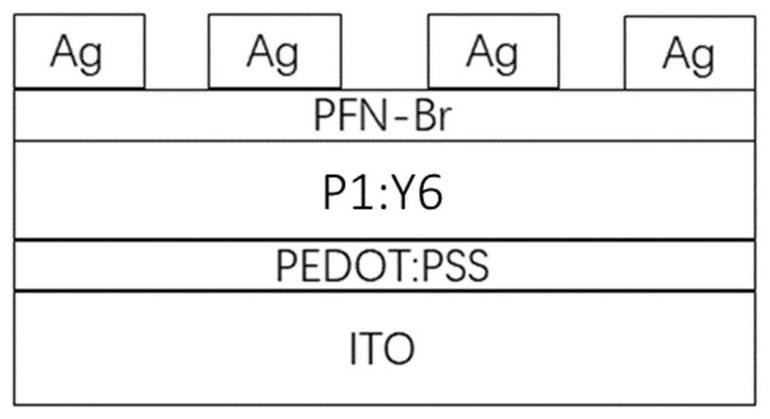
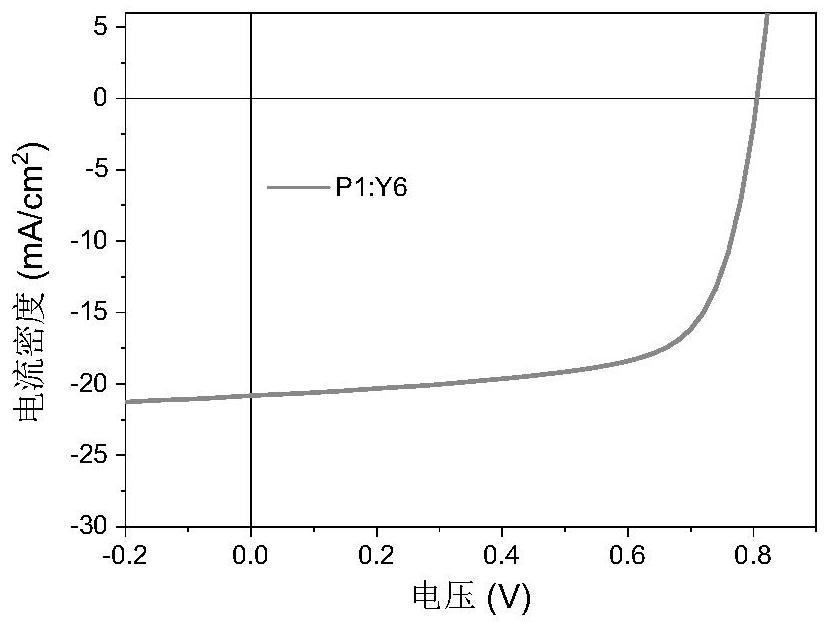
Polythiophene as well as preparation method and application thereof
A technology of polythiophene and polymerization reaction, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc. It can solve problems such as difficulty in regulation and backward device performance, and achieve low manufacturing cost, improved device performance, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 3-cyanothiophene-based monomer M1:
[0037]
[0038] The synthetic route is as follows:
[0039]
[0040] (1) Synthesis of intermediate 3
[0041] Intermediate 1 (2.00g, 7.50mmol) was added to a dry reaction flask, reactant 2 (13.43g, 22.48mmol) was added, catalyst Pd (PPh 3 ) 4 (0.43g, 0.37mmol), in N 2 Add an appropriate amount of anhydrous N,N-dimethylformamide to dissolve under protection, and react overnight at 80°C. The reaction was quenched by adding water, then extracted, concentrated, dried, and separated by column chromatography to obtain a bright yellow oily product with a yield of 93%.
[0042] (2) Synthesis of monomer M1
[0043] Add intermediate 3 (2.50g, 3.46mmol) into a dry reaction flask, add appropriate amount of chloroform and acetic acid to dissolve, and slowly add N-bromosuccinimide (1.23g , 6.92mmol), slowly rise to room temperature, thin-layer chromatography (TLC) monitors the reaction process, after the reaction is comp...
Embodiment 2
[0045] Preparation of 3-cyanothiophene-based monomer M2:
[0046]
[0047] The synthetic route is as follows:
[0048]
[0049] (1) Synthesis of Intermediate 5
[0050]Intermediate 1 (1.00g, 3.75mmol) was added to a dry reaction flask, reactant 4 (7.35g, 11.24mmol) was added, catalyst Pd (PPh 3 ) 4 (0.17g, 0.15mmol), in N 2 Add an appropriate amount of anhydrous N,N-dimethylformamide to dissolve under protection, and react overnight at 80°C. The reaction was quenched by adding water, then extracted, concentrated, dried, and separated by column chromatography to obtain a bright yellow oily monomer with a yield of 95%.
[0051] (2) Synthesis of monomer M2
[0052] Add intermediate 5 (1.97g, 2.36mmol) into a dry reaction flask, add appropriate amount of chloroform and acetic acid to dissolve, and slowly add N-bromosuccinimide (0.84g , 4.72mmol), slowly rose to room temperature, TLC monitored the degree of reaction, quenched with water after the completion of the react...
Embodiment 3
[0054] Preparation of 3-cyanothiophene-based monomer M3:
[0055]
[0056] The synthetic route is as follows:
[0057]
[0058] (1) Synthesis of Intermediate 7
[0059] In a 250mL double necked round bottom flask, under N 2 Under protection, magnesium turnings (2.4 g, 100 mmol) and a small amount of iodine were dissolved in 150 mL of anhydrous THF. Slowly add 5-bromo-1-pentene (11.9g, 80mmol), stir for 20 minutes, then raise the temperature to 80°C and continue the reaction for 2.5 hours, then cool to 0°C and slowly add the above reaction solution dropwise to compound 6 (12.2 g,75mmol), Ni(dppp) 2 Cl 2 (100mg) in a round bottom flask. The reaction was continued for 12 hours at room temperature. After the reaction was completed, it was quenched by adding water, then extracted, concentrated, dried, and separated by column chromatography to obtain a light yellow oily monomer with a yield of 80%.
[0060] (2) Synthesis of Intermediate 8
[0061] In a 250 mL two-neck ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com