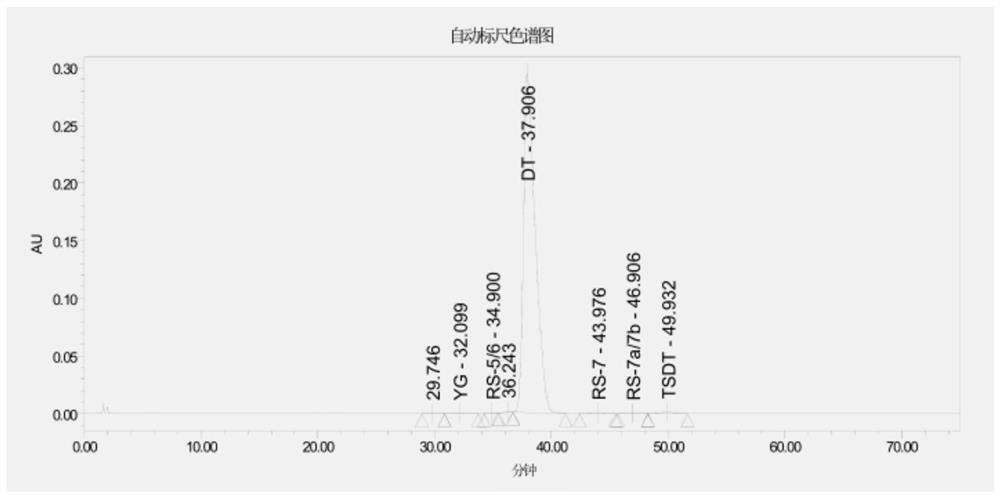
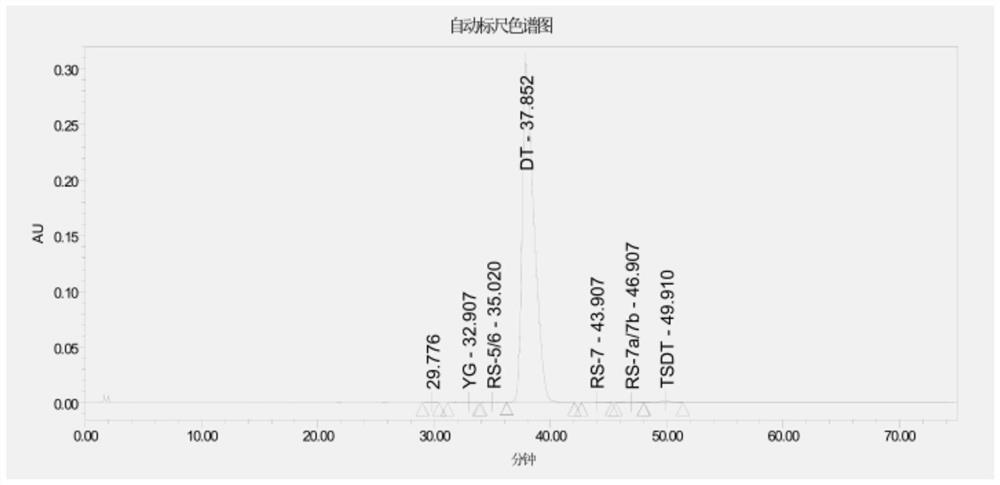
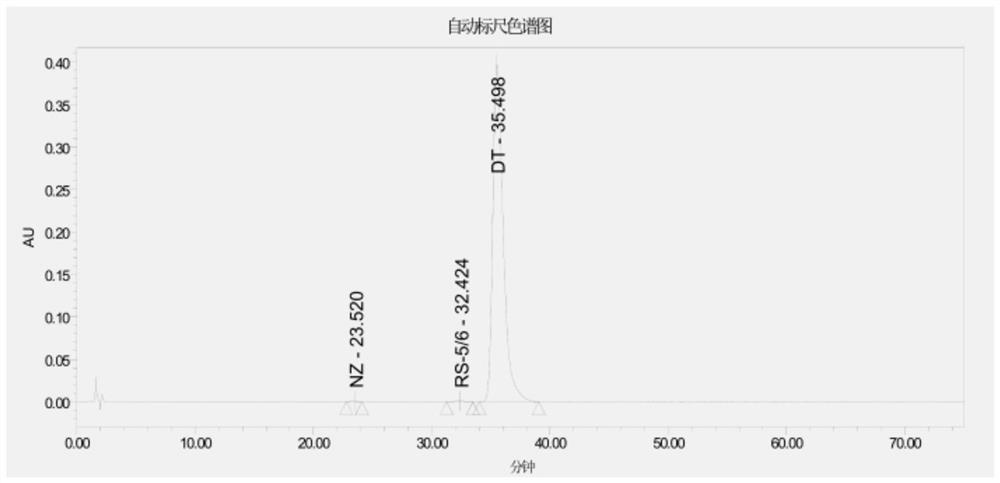
Daptomycin for injection and preparation method thereof
A technology of daptomycin and dehydrated daptomycin, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulations, peptide/protein components, etc., can solve problems such as unfavorable industrial scale production, low product content, poor clarity, etc. problem, to achieve the effect of reducing solvent consumption, high purity and fast reconstitution speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Step (1): Introduce 30L of daptomycin filtrate with a titer of 1026u / mL into an ion exchange resin (about 4L in volume) with a diameter of about 10cm for enrichment and adsorption, wash with pure water, and elute with 0.01M NaCl, Finally, desorb with 1M NaCl; collect the desorbed solution with a titer greater than 1000u / mL and combine them. The volume of the final solution was 14L, the content of daptomycin was 1550u / mL, and the yield was 70.5%.
[0058] Step (2): Ultrafiltration of the analysis solution obtained in step (1) with a 30KD ultrafiltration membrane, and the above operating temperatures are all controlled below 20°C. 22 L of filtrate was obtained, with a titer of 940u / mL. The yield was 95.3%.
[0059] Step (3): The filtrate obtained in step (2) is introduced into a macroporous adsorption resin (about 3 L in volume) with a diameter of about 8 cm for adsorption, washed with pure water, eluted with 15% ethanol, and then washed with 35% ethanol The ethanol wa...
Embodiment 2
[0069] Step (1): Introduce 30L of daptomycin filtrate with a titer of 1026u / mL into an ion exchange resin (about 4L in volume) with a diameter of about 10cm for enrichment and adsorption, wash with pure water, and elute with 0.5M NaCl, Finally, desorb with 5M NaCl; collect the desorbed solution with a titer greater than 1000u / mL and combine them. The volume of the final solution was 12L, the content of daptomycin was 1784u / mL, and the yield was 71.4%.
[0070] Step (2): Ultrafiltration of the analysis solution obtained in step (1) with a 30KD ultrafiltration membrane, and the above operating temperatures are all controlled below 20°C. 20L of the filtrate was obtained with a titer of 1007u / mL. The yield was 94.1%.
[0071] Step (3): import the filtrate obtained in step (2) into a macroporous adsorption resin (about 3 L in volume) with a diameter of about 8 cm for adsorption, wash with pure water, elute with 25% ethanol, and then use 45 % ethanol was used for desorption, the ...
Embodiment 3
[0081] Step (1): Introduce 30L of daptomycin filtrate with a titer of 1026u / mL into an ion exchange resin (about 4L in volume) with a diameter of about 10cm for enrichment and adsorption, wash with pure water, and elute with 0.32M NaCl, Finally, desorb with 2.7M NaCl; collect the desorbed solution with a titer greater than 1000u / mL and combine them. The volume of the final analysis solution was 12L, the content of daptomycin was 1820u / mL, and the yield was 71.9%.
[0082] Step (2): Ultrafiltration of the analysis solution obtained in step (1) with a 30KD ultrafiltration membrane, and the above operating temperatures are all controlled below 20°C. Obtain 20L of filtrate, titer is 1020u / mL. The yield was 93.4%.
[0083] Step (3): The filtrate obtained in step (2) is introduced into a macroporous adsorption resin (about 3 L in volume) with a diameter of about 8 cm for adsorption, washed with pure water, eluted with 19% ethanol, and then washed with 40% ethanol The ethanol was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com