Method for determining concentration of ferrovanadium ions in mixed solution
A mixed solution and ion concentration technology, applied in the field of electrolysis, can solve the problems of expensive instruments, inability to test mixed solutions of iron and vanadium, and inability to obtain the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0073] Solution 1: Prepare tetravalent vanadium (VO) with 3mol / L sulfuric acid 2+ ) Solution 1 with a concentration of 1.0 mol / L, and determine the concentration with a potentiometric titrator.
[0074] Solution 2: Prepare ferrous iron (Fe) with 3mol / L sulfuric acid 2+ ) Solution 2 with a concentration of 1.0 mol / L, and determine the concentration with a potentiometric titrator.
[0075] Solution 3: prepare ferric iron (Fe) with 3mol / L sulfuric acid 3+ ) Solution 3 with a concentration of 1.0 mol / L, and determine the concentration with a potentiometric titrator.
[0076] Solution 4: Electrolyze solution 1 to divalent vanadium (V 2+ ) concentration is 0.7mol / L, trivalent vanadium (V 3 + ) concentration is 0.3mol / L.
[0077] Solution 5: Prepare KMnO with a molar concentration of 0.02mol / L 4 solution as standard solution V, and for KMnO 4 The solution was calibrated with an accurate concentration of 0.0183mol / L.
[0078] Solution 6: Prepare a mixed buffer solution with a...
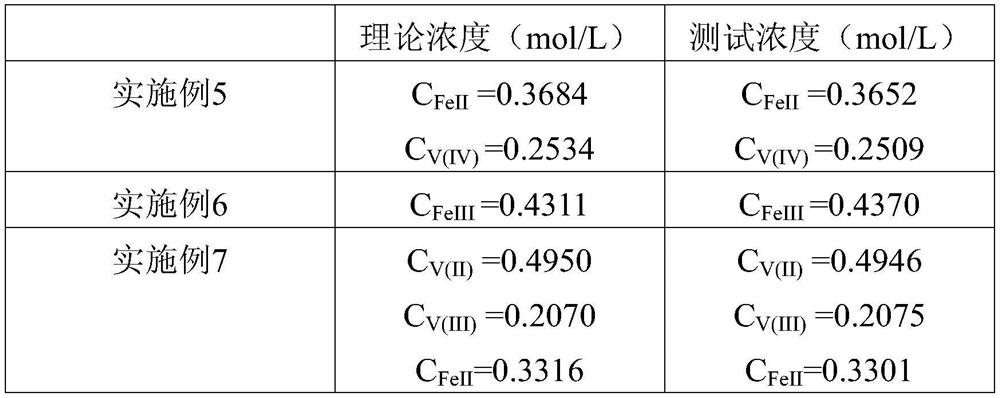
Embodiment 1
[0081] Take 0.5mL (solution one) tetravalent vanadium and 50ml (solution six) sulfuric acid phosphate buffer in a 100mL beaker. Using a fully automatic potentiometric titrator, drop (solution five) 0.0183mol / L of KMnO into the beaker 4 Standard solution, record the volume of potassium permanganate solution consumed when the first potential jump point is obtained, 5.5390mL. Continue to drop KMnO 4 solution, the solution is purple, indicating that all vanadium exists in the pentavalent state, KMnO 4 Excess solution.
[0082] The concentration of tetravalent vanadium is
[0083] C V(IV) = 5 x 5.5390 mL x 0.0183 mol / L ÷ (0.5 mL) = 1.0136 mol / L.
Embodiment 2
[0085] Take 0.5mL (solution two) of ferrous iron and 50ml (solution six) of sulfuric acid phosphate buffer in a 100mL beaker. Using a fully automatic potentiometric titrator, drop (solution five) 0.0183mol / L of KMnO into the beaker 4Standard solution, record the volume of potassium permanganate solution consumed when the first potential jump point is obtained: 6.0400mL. Continue to drop KMnO 4 solution, the solution is purple, indicating that all iron exists in the trivalent state, KMnO 4 Excess solution.
[0086] The concentration of ferrous iron is C FeII = 5 x 6.04 mL x 0.0183 mol / L ÷ (0.5 mL) = 1.1053 mol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com