Low-renal-toxicity protein-ferric oxide composite nano magnetic resonance contrast agent as well as preparation method and application thereof
A magnetic resonance contrast agent, iron oxide technology, applied in the field of medical materials, can solve the problem of unverified contrast effect of magnetic resonance imaging nano-contrast agent, large inorganic core particle size of magnetic resonance imaging nano-contrast agent, limited angiography effect and biological safety. problems, such as low cost, high synthesis efficiency, low r1 value and saturation magnetization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation and characterization of low nephrotoxic protein - iron oxide composite nano magnetic resonance contrast agent
[0052] 1) Preparation of low nephrotoxic protein-iron oxide composite nano magnetic resonance contrast agent
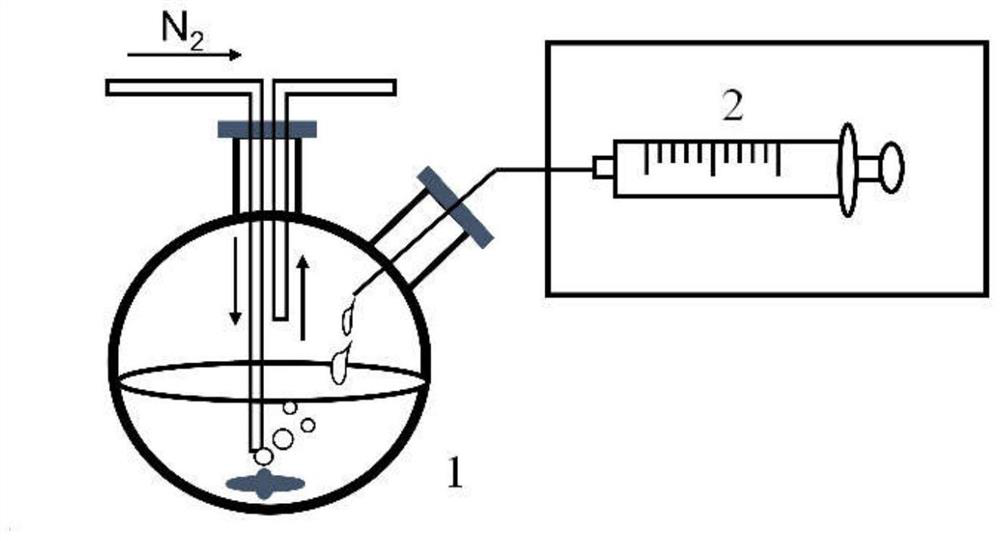
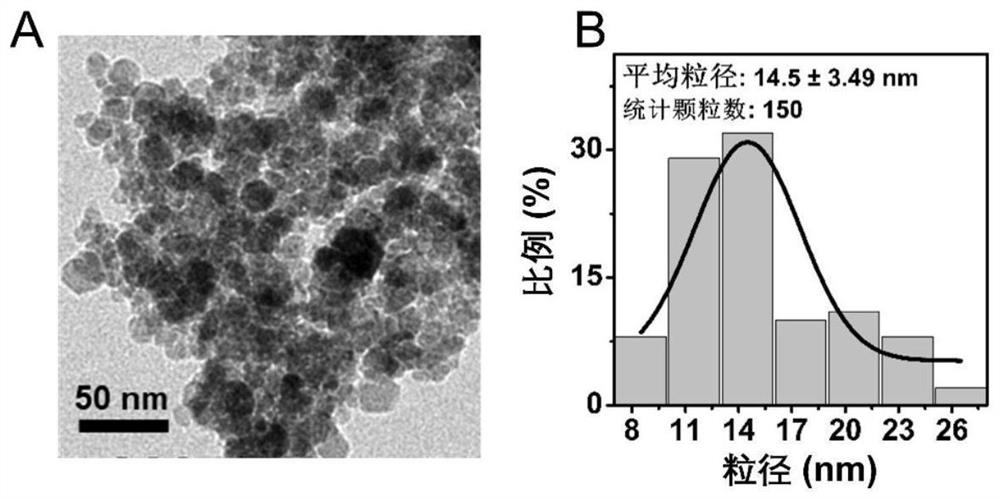
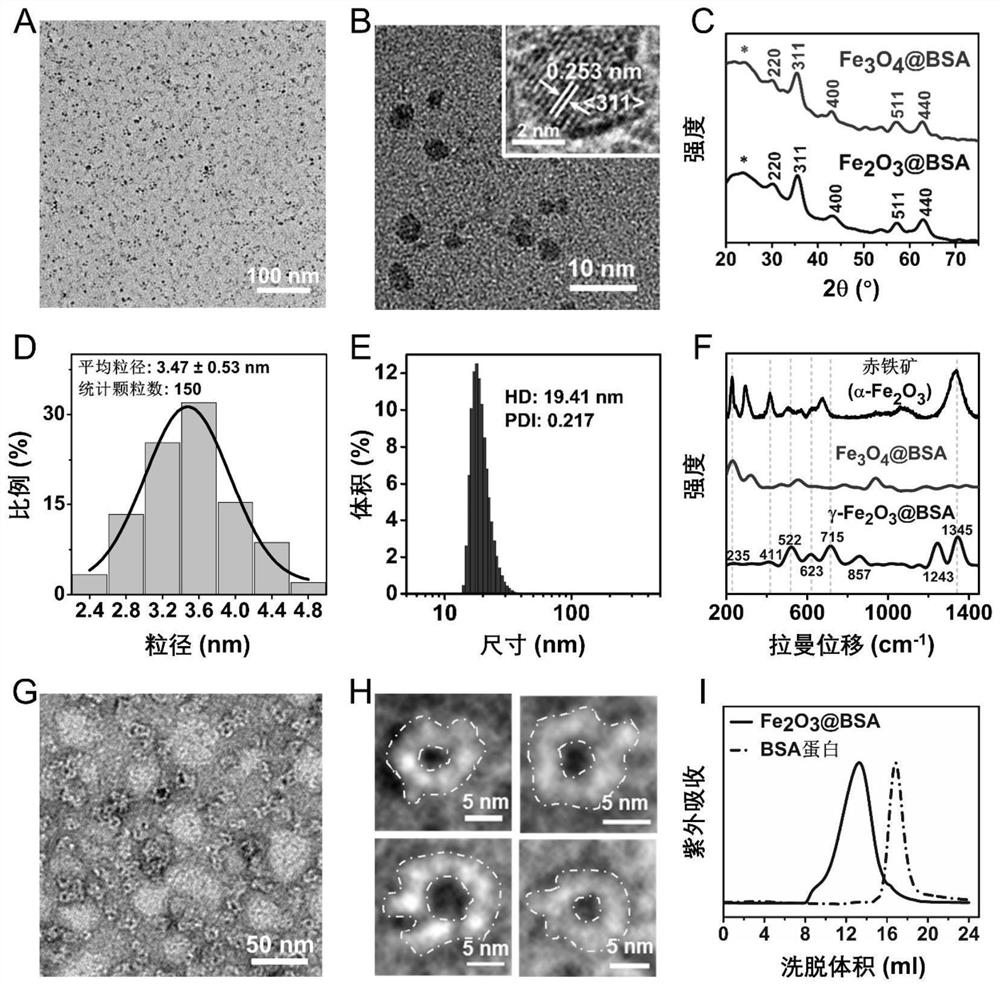
[0053] This embodiment uses a chemical co-precipitation method to prepare a variety of Fe having different Fe and BSA molar proportion relationships to Fe 2 O 3Magnetic resonance contrast agent for inorganic cores (i.e. BSA-wrapped Fe 2 O 3 , hereinafter referred to as Fe 2 O 3 @BSA nanoparticles), also prepared to Fe 3 O 4 Magnetic resonance contrast agent for inorganic cores (i.e. BSA-wrapped Fe 3 O 4 , hereinafter referred to as Fe 3 O 4 @BSA nanomelets), Fe 2 O 3 @BSA nanoparticles and Fe 3 O 4 @BSA nanoparticles are collectively referred to as iron oxide @BSA nanoparticles, and the specific operation steps are:
[0054] 1.1, with ultrapure water to prepare 80mg / ml BSA solution (diluted to obtain 40mg / ml, 20mg / ml, 10mg / ...
Embodiment 2
[0085] Example 2: Preparation of magnetic resonance imaging nano contrast agent
[0086] The embodiment is intended to utilize a variety of having different Fe 2+ and Fe 3+ Molar ratio of iron salt solution to prepare magnetic resonance imaging nano contrast agent, which differs only from the preparation method provided in Example 1 is: preparation of iron salt solution according to the table 3 below (wherein FeSO 4 Solution and FeCl 3 The total amount of the solution added is 2 ml for example), followed by the addition of BSA solution in accordance with the molar ratio of Fe to BSA is 165:1, and then the NaOH solution (in accordance with steps 1.1 to 1.6 in the preparation method provided in Example 1) or sequentially added NaOH solution and excess hydrogen peroxide solution (according to steps 1.1 to 1.11 in the preparation method provided in Example 1), and finally a series of iron oxide @BSA nanoparticles were prepared.
[0087] Table 3: With different Fe 2+ and Fe 3+ Molar th...
Embodiment 3
[0092] Example 3: Fe 2 O 3 in vivo MR imaging of @BSA nanoparticles
[0093] This embodiment is Fe prepared in the above example 1 2 O 3 @BSA4 nanoparticles as an example of an in vivo MR contrast analysis of the nanoparticles provided by the present invention, including the following operations.
[0094] 3.1. Fe is passed through the tail vein 2 O 3 @BSA4 nanoparticles or Gd-DTPA (0.15 mmol Fe / Gd per kilogram of rat body weight) are injected intravenously into 6-week-old male Sprague Dawley (SD) rats (purchased from SMOX, with an average weight of 200 g, all rats are cared for according to the guidelines outlined in the Laboratory Animal Care and Use Guidelines).
[0095] 3.2. Intraperitoneal injection of 5% chloral hydrate anesthetized rats, check the sagittal slices (i.e., MRI scan rat results) before and after injection for 2 min, 5 min, 15 min, 30 min, 45 min, 60 min. MR contrast images were obtained using a 3T clinical MRI scanner, in which the T1-weighted image was acquired...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com