Method for synergistically strengthening sulfuric acid leaching of chalcopyrite
A chalcopyrite and leaching technology, applied in the field of hydrometallurgy, can solve the problems of limited improvement of surfactants, and achieve the effects of low price, elimination of passivation, and strong application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In the present embodiment, the method for synergistically strengthening sulfuric acid leaching of chalcopyrite is carried out according to the following steps:
[0047] (1) Pyrite-type chalcopyrite is crushed and ground to -0.045mm as a leaching ore sample;
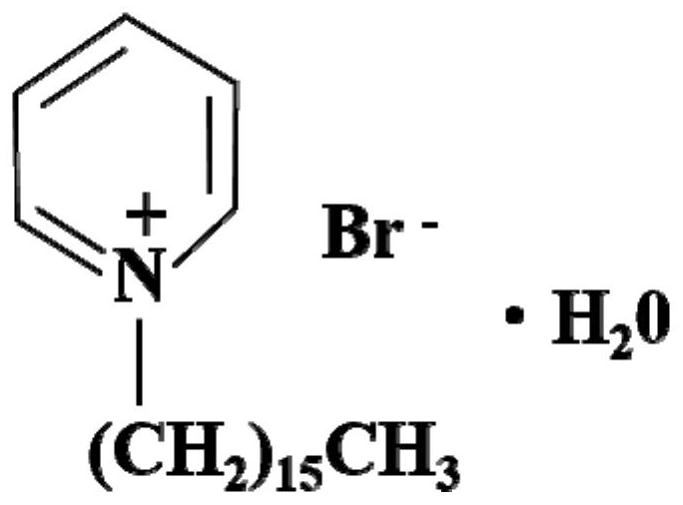
[0048] (2) Take 1g of ore sample in a 150mL Erlenmeyer flask, add 100mL Fe-free 2+ solution, followed by the addition of 0.5mol / L NH 4 Cl and 60mg / L CPB were placed in a conical flask, and the initial pH of the leachate was adjusted to 2.0 with a concentration of 5mol / L dilute sulfuric acid;
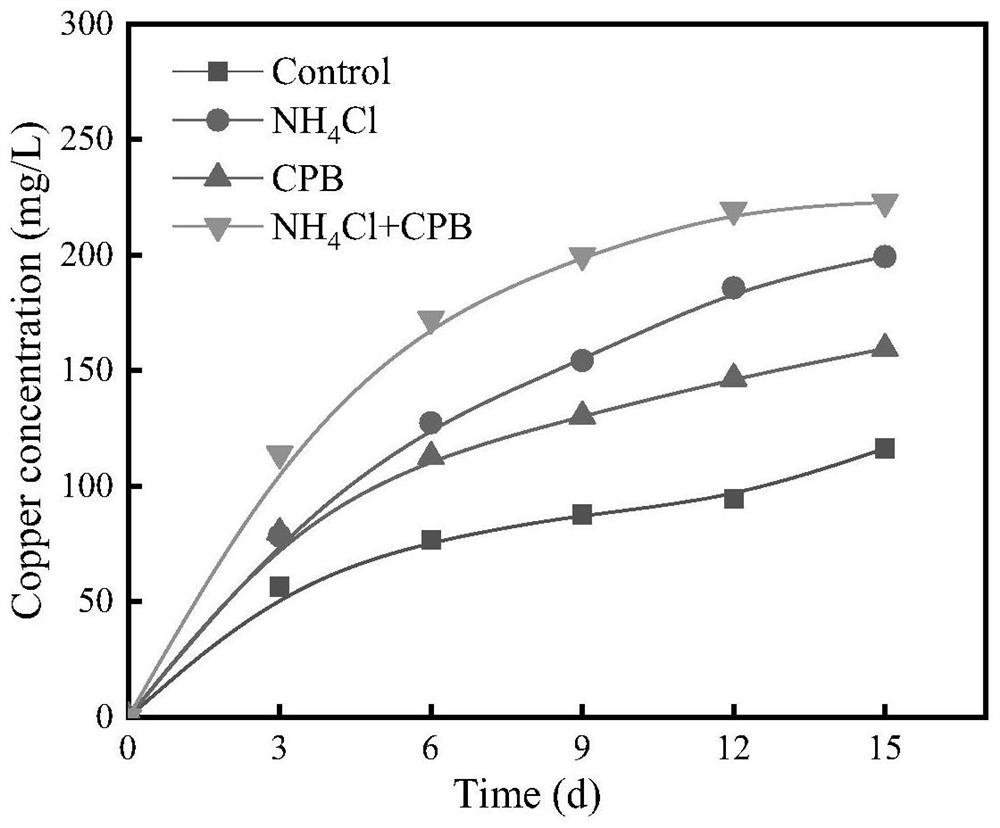
[0049] (3) Place the above leaching system in an air bath constant temperature oscillator at 30°C and 160r / min for shake flask leaching for 15 days, take samples regularly every 3 days and centrifuge to remove precipitated substances, then take the supernatant to detect the content of copper ions , replenish the evaporated water with deionized water, and supplement the amount of liquid consumed by the test with the correspo...
Embodiment 2
[0055] In the present embodiment, the method for synergistically strengthening sulfuric acid leaching of chalcopyrite is carried out according to the following steps:
[0056] (1) Crushing and grinding the pyrite-type chalcopyrite to -0.038mm or -0.045mm particle size level as a leaching ore sample;
[0057] (2) Take 1g of ore samples with particle size of -0.038mm in a 150mL Erlenmeyer flask, add 100mL deionized water, and then add 0.5mol / L NH 4 Cl, 120mg / L CPB and 0.5mol / L NH 4 Cl+120mg / L CPB, using dilute sulfuric acid with a concentration of 5mol / L to adjust the initial pH of the leaching solution to 1.5, and finally place the above leaching system in an air bath constant temperature oscillator at 50°C and 200r / min for shake flask leaching for 10 days;
[0058] (3) Taking NaCl and KCl two common chloride salts reported in the literature as a control, take 1g of ore samples with a particle size of -0.045mm in a 150mL conical flask, add 4.5g / L Fe 2+ As an oxidizing agent, ...
Embodiment 3
[0063] In the present embodiment, the method for synergistically strengthening sulfuric acid leaching of chalcopyrite is carried out according to the following steps:
[0064] (1) Porphyry-type chalcopyrite is crushed and ground to -0.045mm as a leached ore sample;
[0065] (2) Take 1g of ore sample in a 150mL Erlenmeyer flask, add 100mL Fe-free 2+ or 4.5g / L Fe 2+ solution, followed by adding 1mol / L NH 4 Cl and 30mg / L CPB were placed in a conical flask, and the initial pH of the leachate was adjusted to 2.0 with a concentration of 5mol / L dilute sulfuric acid;
[0066] (3) Anionic surfactant - sodium dodecyl sulfonate (SDS) and non-ionic Tween surfactant - polyoxyethylene sorbitan monolaurate (Tween20) two surfaces The active agent is used as a reference, and under the condition that the dosage is 30mg / L, in the absence of Fe 2+ In the sulfuric acid leaching system, with 1mol / L NH 4 Cl combination, to investigate the effect of different combinations of additives on the lea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com