Ionic liquid of solvated organic lithium borate, preparation method and application of ionic liquid, and lubricating oil
A technology of organic lithium borate and lithium borate salt, applied in the field of lubricating oil, can solve problems such as equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides the preparation method of organic borate lithium salt described in above-mentioned technical scheme, comprises the following steps:
[0040] The organic borate lithium salt is obtained by mixing alkyl dicarboxylic acid, boric acid, lithium hydroxide and an organic solvent and performing condensation reaction.
[0041] In the present invention, the alkyl group in the alkyl dicarboxylic acid is a linear alkyl group with 2 to 8 carbon atoms, more preferably 2 or 3; the alkyl dicarboxylic acid may specifically include ethylene dicarboxylic acid acid or malonic acid, more preferably malonic acid.
[0042] In the present invention, the organic solvent preferably includes tetrahydrofuran, toluene or xylene, more preferably tetrahydrofuran or toluene, still more preferably toluene. In the present invention, the toluene is preferably anhydrous toluene.
[0043] In the present invention, the molar ratio of the alkyl dicarboxylic acid, boric a...
Embodiment 1
[0072] Dissolve 0.04 mol of acetic acid, 0.02 mol of boric acid and 0.02 mol of lithium hydroxide in anhydrous toluene, condense in an oil bath at 110°C (with reflux and stirring at a speed of 450r / min) for 12 hours, and air-cool the solution after the condensation reaction to Rotary evaporation at 25°C for 30min at 65°C to obtain a solid; wash the obtained solid with n-hexane and dry at 60°C for 12h to obtain white powder lithium bis(oxalate)borate.
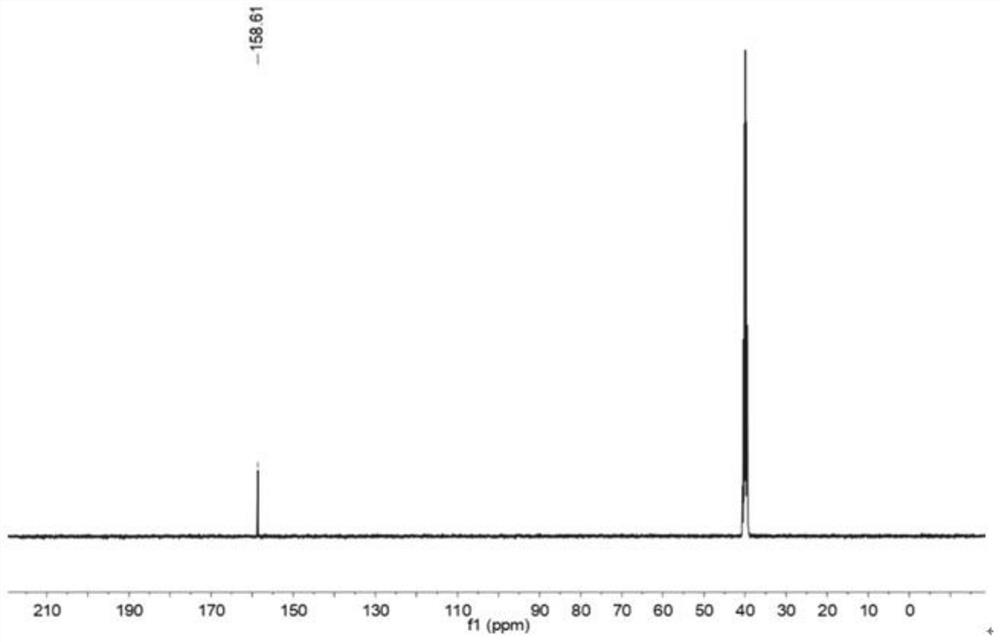
[0073] Carry out nuclear magnetic resonance detection to the two (oxalic acid) lithium borate that prepares, obtain carbon spectrogram such as figure 1 shown. The detection result of the carbon spectrogram is 13 C NMR (101MHz, DMSO-d 6 ) δ158.61.
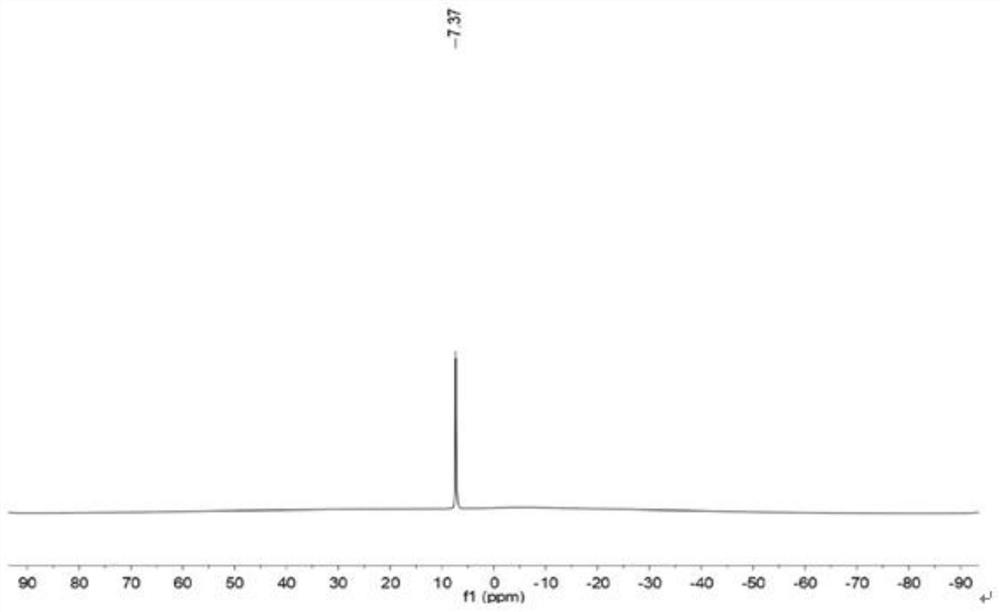
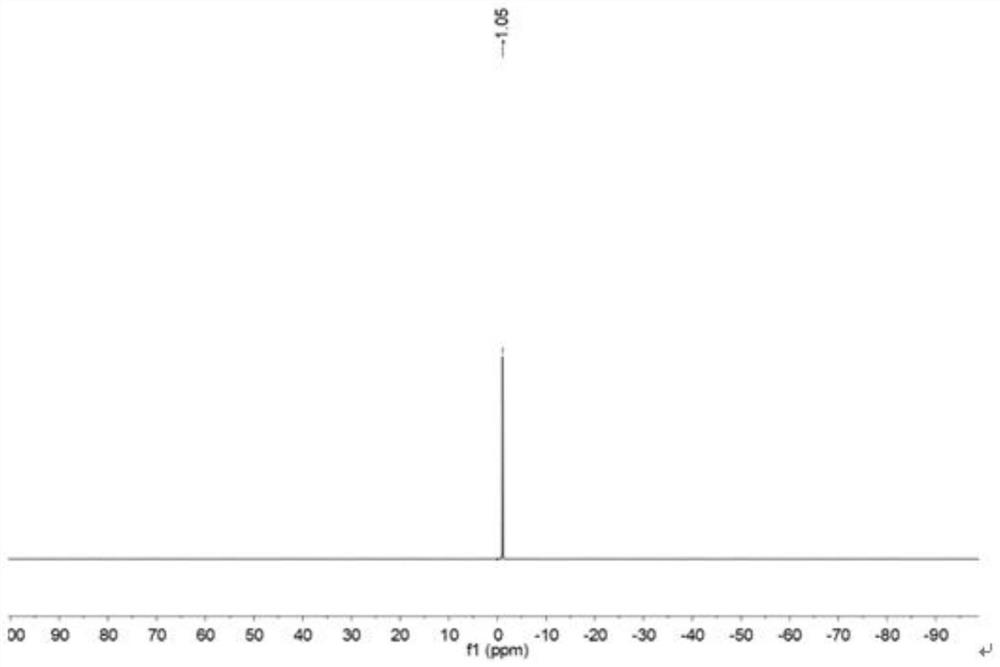
[0074] The prepared bis(oxalate) lithium borate is detected by nuclear magnetic resonance, and the boron spectrogram is obtained as figure 2 shown. The detection result of the boron spectrum is 11 B NMR (128MHz, DMSO-d 6 )δ7.37. 7 Li NMR (155MHz, DMSO-d 6 )δ-1.05.
[0075] ...
Embodiment 2
[0077] Lithium organoborate was prepared according to the method in Example 1, except that oxalic acid was replaced by malonic acid to prepare lithium bis(malonate)borate.
[0078] The prepared double (malonate) lithium borate is detected by nuclear magnetic resonance, and the hydrogen spectrum is obtained as image 3 shown. The detection result of the hydrogen spectrogram is 1 H NMR (400MHz, DMSO-d 6 )δ3.25(s,4H).
[0079] The prepared bis(malonate) lithium borate is detected by nuclear magnetic resonance, and the carbon spectrum is obtained as Figure 4 shown. The detection result of the carbon spectrogram is 13 C NMR (101MHz, DMSO-d 6 ) δ168.86, 42.40.
[0080] The prepared bis(malonate) lithium borate is detected by nuclear magnetic resonance, and the boron spectrogram is obtained as Figure 5 shown. The detection result of the boron spectrum is 11 B NMR (128MHz, DMSO-d 6 )δ3.43,-87.88. 7 Li NMR (155MHz, DMSO-d 6 )δ-1.05.
[0081] It can be known from the NMR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com