Target membrane micromolecule and preparation method and application thereof
A technology of small molecules and target membranes, applied in chemical instruments and methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problem of low yield of near-infrared fluorescent probes and the inability to achieve reliability in the second near-infrared region Fluorescent labeling and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
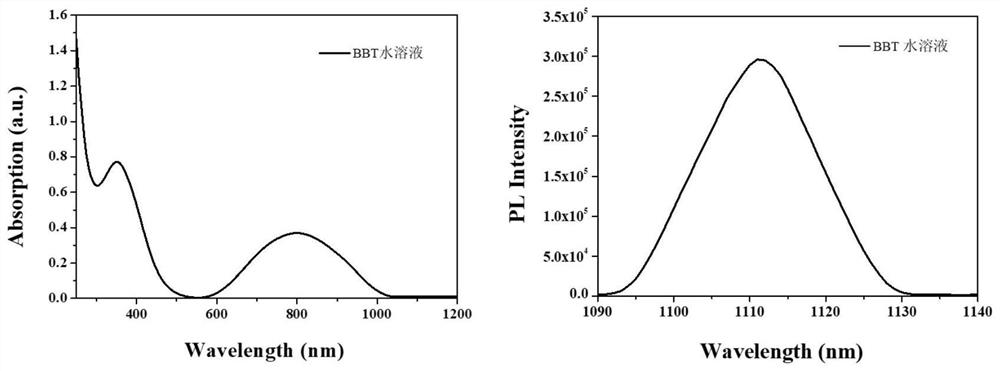
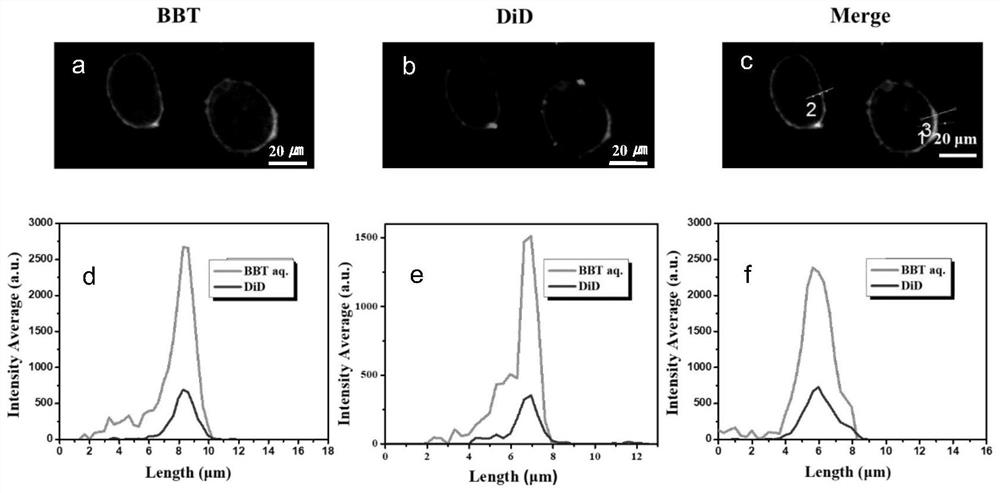
Image
Examples
Embodiment 1
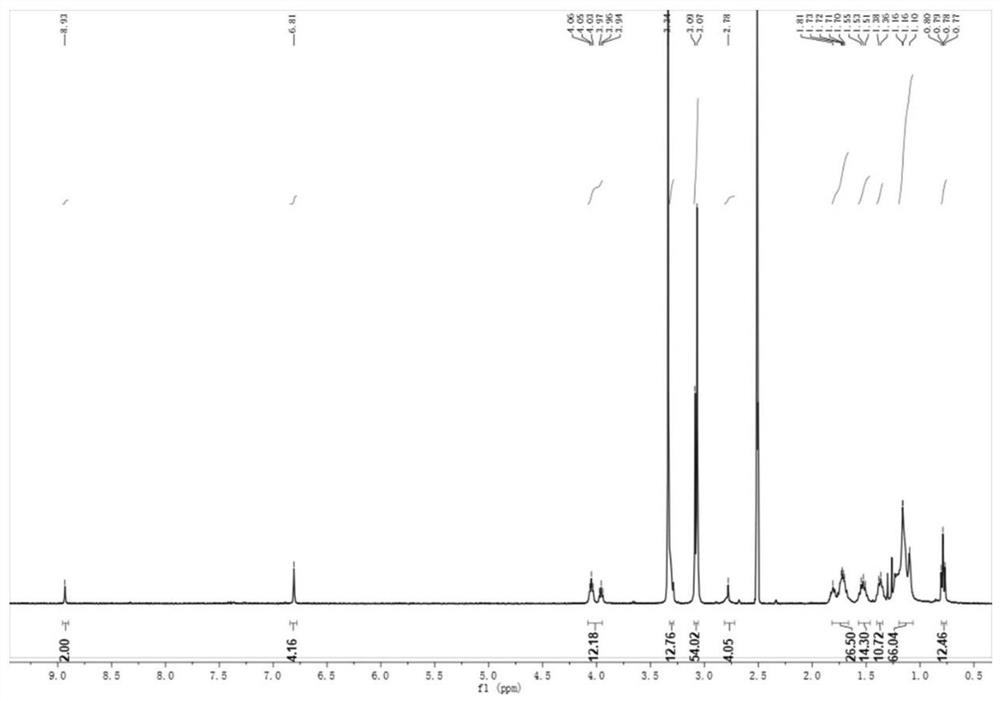
[0028] Example 1: Preparation of Intermediate 1
[0029]
[0030] Dissolve 1-bromo-3,4,5-trimethoxybenzene (5.0 g, 1.0 eq) in 100 mL of dichloromethane, evacuate nitrogen and cool it to -78 °C in a dry ice / acetone bath for about 30 min, Boron tribromide (20.0 g, 4.0 eq) was added dropwise and incubated at -78 °C for 30 min, then the solution was slowly warmed to 40 °C. After 24 h of reaction, saturated aqueous ammonium chloride solution was added to quench the reaction, the mixture was filtered and washed with ethyl acetate, then the filtrate was extracted with ethyl acetate, the organic phase was dried over magnesium sulfate and separated and purified using silica gel column chromatography (ethyl acetate). : n-hexane=3:10) to obtain Intermediate 1.
Embodiment 2
[0031] Example 2: Preparation of Intermediate 2
[0032]
[0033] Potassium carbonate (4.0 g, 6.0 eq) and intermediate 1 were dissolved in 100 mL of acetone, and nitrogen was sparged under vacuum, and 5-bromo-1,2,3-phloroglucinol (1.0 g, 1.0 eq) was added, and the mixture was heated at 90 °C. Heat to reflux and stir for 24h. After the reaction was completed, it was extracted with dichloromethane and separated and purified by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain Intermediate 2.
Embodiment 3
[0034] Example 3: Preparation of Intermediate 3
[0035]
[0036] Intermediate 2 (1.0g, 1.0eq) and potassium acetate (0.4g, 2.5eq), pinacol biboronate (0.6g, 1.3eq), catalyst 1 × 1' bis(triphenylphosphine) dimethylene Ferric palladium chloride (24.0 mg) was added to the reaction flask, 20.0 mL of 1,4-dioxane was added, and nitrogen was sparged under vacuum, and the reaction was carried out at 100° C. for 2 h. After completion of the reaction, extract with dichloromethane, spin dry, and use silica gel column chromatography for separation and purification (petroleum ether:dichloromethane=10:2) to obtain Intermediate 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com