A fluorescent probe that can be used for imaging malondialdehyde in living cells and its preparation method
A fluorescent probe and malondialdehyde technology, which is applied in the field of fluorescent dyes, can solve the problems of low detection limit, inability to use imaging, and cumbersome pretreatment of detection samples, so as to avoid serious self-absorption, short response time, and excellent selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic route of fluorescent probes MDAP-1, MDAP-2, and MDAP-3 that can be used for imaging malondialdehyde in living cells is shown in the following reaction formula:
[0033]
[0034] ①The intermediates (3a, 3b, 3c) were synthesized by Buchwald-Harwig amino coupling method
[0035] A. Synthesis of Compound 3a
[0036] Weigh 300 mg of 6-bromo-2-propyl-1H-benzo[de]isoquinoline-1,3(2H)-dione (compound 1, 2.0 mmol) (see J.Org. Chem.2013,78,3980-3988) into a 50 ml two-necked flask, then successively added 370 mg of methyl 5-amino-2-nitrobenzoate (compound 2a, 1.89mmol) (the synthesis of compound 2a see Tetrahedron. Lett.2005, 46, 7477-7481), 42 mg Pd 2 (dba) 3 (0.046mmol) and 66 mg (±)-2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl (BINAP), stirred and mixed, degassed, and added 18 ml of freshly distilled Toluene, heated to boiling, reacted overnight under reflux conditions, and cooled to room temperature. After the solvent was removed under reduced pressure, i...
Embodiment 2
[0053] Example 2 Fluorescence response of fluorescent probes MDAP-1, MDAP-2, MDAP-3 and malondialdehyde (MDA)
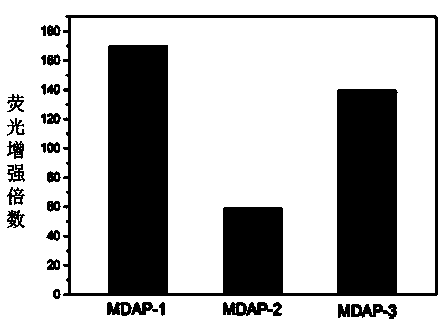
[0054] Prepare the stock solution (100mM) of fluorescent probes MDAP-1, MDAP-2 and MDAP-3 in DMSO, take a small amount and add it to the EP tube, dilute it with PBS buffer solution (10mM, pH 7.4), add a certain amount of MDA solution, Then dilute with DMSO and PBS to a final concentration of probe of 10 μM and a final concentration of MDA of 1 mM (solvent composition at this time: DMSO:PBS=1:9, volume ratio, pH 7.4). The fluorescent response of the EP tubes was measured after incubation at 37°C for four hours. Taking the maximum excitation wavelength as 370nm, the emission wavelengths of the three compounds are all around 550-553nm, and the Stokes shifts are all around 180nm, which is quite large compared to ordinary fluorescent probes. It can effectively avoid the interference of excitation light on emission, which is very beneficial for cell imaging; the fluoresce...
Embodiment 3
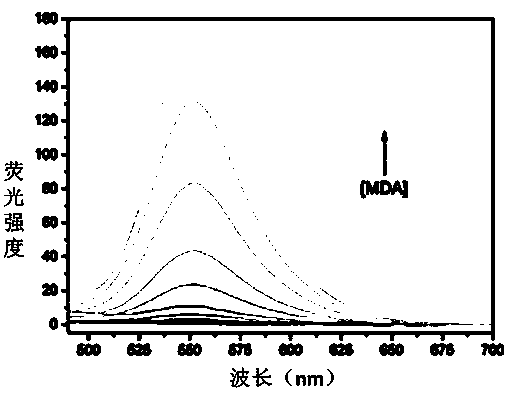
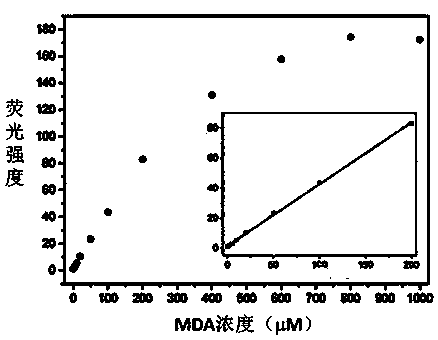
[0055] Embodiment 3 The correlation between the amount of fluorescent probe MDAP-1 and malondialdehyde (MDA)
[0056] Take a small amount of the DMSO stock solution of MDAP-1 in an EP tube, dilute it with PBS buffer (10mM, pH 7.4), add a certain amount of MDA solution, and then dilute it with PBS and DMSO until the final concentration of the probe is 10μM. The concentrations were 0, 2, 5, 10, 20, 50, 100, 200, 400, 600, 800, 1000 μM, respectively. Incubated at 37°C for 4 hours, and detected the fluorescence intensity of the solution (excitation wavelength: 370nm; emission wavelength: 553nm. The fluorescence diagram of MDAP-1-MDA titration is shown in figure 2 ; The relationship between the fluorescence intensity and MDA is shown in image 3 . Solvent composition: DMSO:PBS=1:9, volume ratio, pH 7.4). The results showed that the fluorescence intensity of MDAP-1 increased with the increase of the concentration, and within the concentration range of 0-200μM, the fluorescence i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com