Levofloxacin tablet and preparation method thereof
A technology for levofloxacin and tablets, which is applied in the field of levofloxacin tablets and its preparation, can solve the problems of unfavorable industrial production, affecting drug efficacy, and high moisture absorption of tablets, and achieves the simple and easy operation of the preparation process, improving bioavailability, Suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
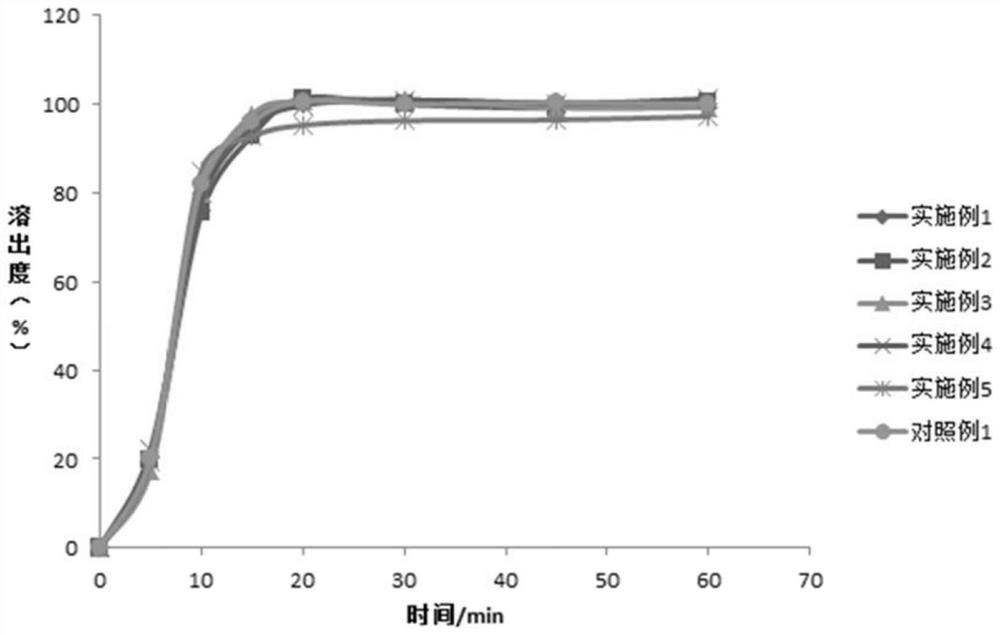
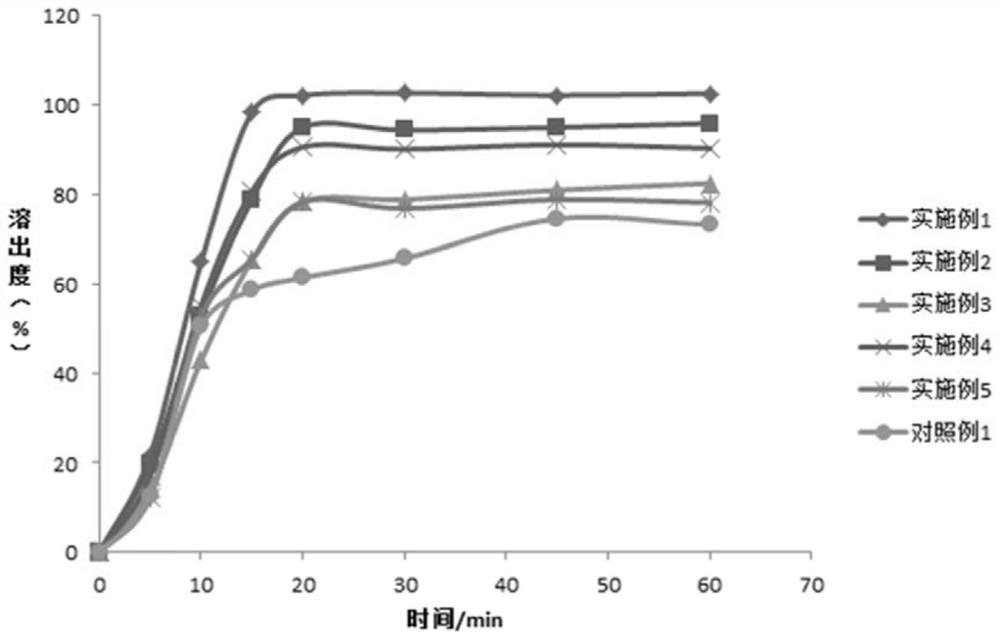
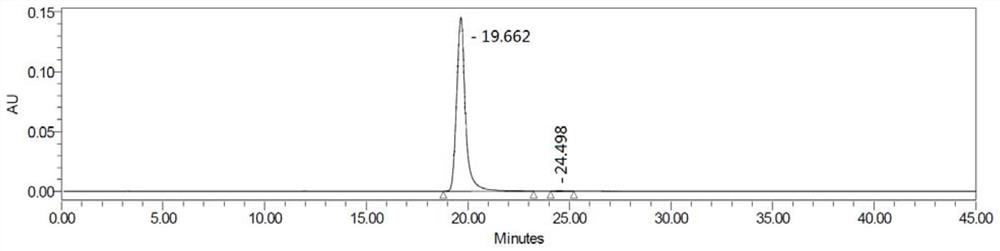
Examples
Embodiment 1-3
[0038] The preparation method of embodiment 1-3
[0039] (1) Levofloxacin hemihydrate, microcrystalline cellulose, crospovidone, and sodium stearyl fumarate are respectively passed through an 80-mesh sieve for subsequent use;
[0040] (2) Sodium dihydrogen phosphate, disodium hydrogen phosphate, and hypromellose of prescription quantity are dissolved in 180g purified water to make binder solution;
[0041] (3) Levofloxacin hemihydrate and microcrystalline cellulose that take recipe quantity are placed in fluidized bed granulator, carry out spray granulation with the binder solution that step (2) makes, dry, then add cross-linking Povidone and sodium stearyl fumarate are mixed evenly and compressed into tablets;
[0042] (4) Coating the base tablet obtained after tableting in step (3) with Opadry to obtain levofloxacin tablets.
Embodiment 4
[0043] The preparation method of embodiment 4
[0044] (1) Levofloxacin hemihydrate, microcrystalline cellulose, crospovidone, and sodium stearyl fumarate are respectively passed through an 80-mesh sieve for subsequent use;
[0045] (2) Sodium dihydrogen phosphate and hypromellose of prescription quantity are dissolved in 180g purified water to make binder solution;
[0046] (3) Levofloxacin hemihydrate and microcrystalline cellulose that take recipe quantity are placed in fluidized bed granulator, carry out spray granulation with the binder solution that step (2) makes, dry, then add cross-linking Povidone and sodium stearyl fumarate are mixed evenly and compressed into tablets;
[0047] (4) Coating the base tablet obtained after tableting in step (3) with Opadry to obtain levofloxacin tablets.
Embodiment 5
[0048] The preparation method of embodiment 5
[0049] (1) Levofloxacin hemihydrate, microcrystalline cellulose, crospovidone, and sodium stearyl fumarate are respectively passed through an 80-mesh sieve for subsequent use;
[0050] (2) Disodium hydrogen phosphate and hypromellose of prescription quantity are dissolved in 180g purified water to make binder solution;
[0051](3) Levofloxacin hemihydrate and microcrystalline cellulose that take recipe quantity are placed in fluidized bed granulator, carry out spray granulation with the binder solution that step (2) makes, dry, then add cross-linking Povidone and sodium stearyl fumarate are mixed evenly and compressed into tablets;
[0052] (4) Coating the base tablet obtained after tableting in step (3) with Opadry to obtain levofloxacin tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com