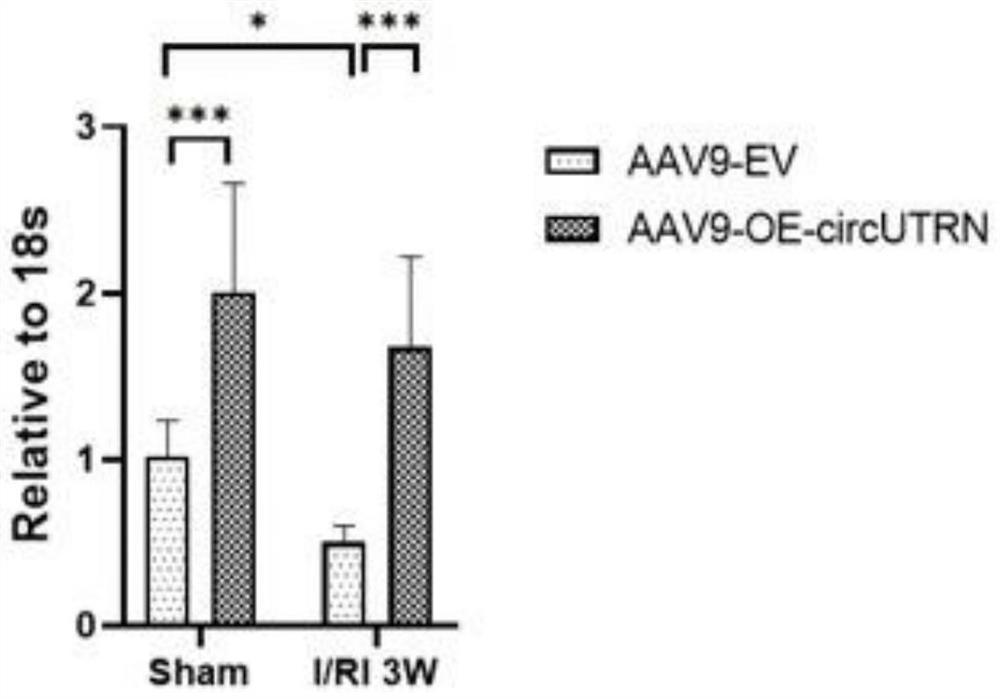
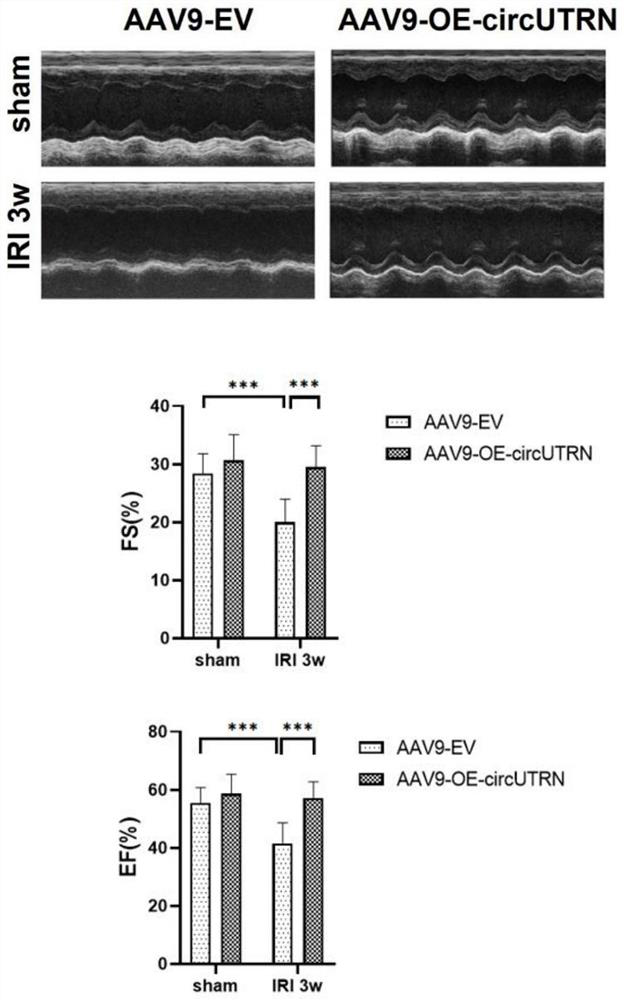
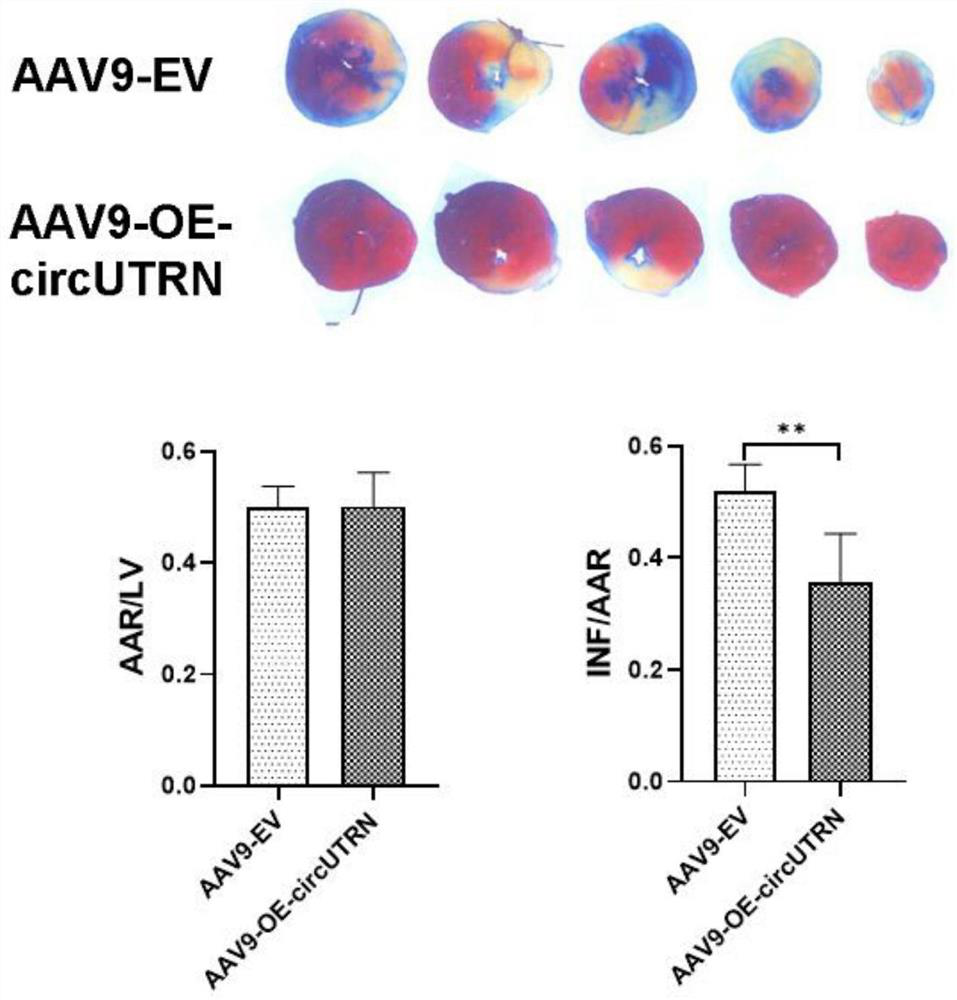
Application of circUTRN in preparation of medicine for treating heart failure, recombinant vector and medicine for treating heart failure
A recombinant carrier and heart failure technology, applied in the field of biomedicine, can solve the problems of bleeding at the puncture point, achieve good therapeutic effect, reduce the infarct size, and inhibit the effect of cardiomyocyte apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A recombinant vector expressing circUTRN is constructed by the following steps:
[0036] 1) Reverse transcribe the total RNA of the mouse heart tissue, perform PCR amplification sequentially, double enzyme digestion, and recover the cDNA sequence (as shown in SEQ ID NO.1) to obtain circUTRN; the nucleoside of the upstream primer of the PCR amplification The acid sequence is as shown in SEQ ID NO.2, specifically as follows: 5'-CGGAATTCTGAAATATGCTATCTTACAGTGGATCTCTTAGAGCTGAATACGACG-3', and the nucleotide sequence of the downstream primer for PCR amplification is as shown in SEQ ID NO.3, specifically as follows:
[0037] 5'-GGAATTCCATATGTCAAGAAAAAATATATTCACCGGTATGCAGTATTCTACCATCGG-3';
[0038] Each 50 μL PCR amplification reaction system consists of the following components: 1 μL of upstream primer (10 μM) (final concentration is 0.2 μM), 1 μL of downstream primer (10 μM) (final concentration is 0.2 μM), 2×TransStart FastPfuPCR SuperMix (full format Gold Biotechnology, Ca...
Embodiment 2
[0048] A recombinant vector expressing circUTRN similar to Example 1, the difference is:
[0049] 1. Replace the downstream primer in step 1) with a downstream primer whose nucleotide sequence is shown in SEQ ID NO.4, specifically as follows:
[0050] 5'-CGGGATCCAGTTGTTCTTACCGGTATGCAGTATTCTACCATCGG-3'; 2, replace NdeI (star selection enzyme) in step 1) with BamHI-HF (star selection enzyme);
[0051] 3. Replace the circular vector in step 2) with the AAV overexpression vector pK5ssAAV-ciR (purchased from Guangzhou Jisai Biotechnology Co., Ltd., catalog number GS0109).
Embodiment 3
[0053] A kind of medicine for treating heart failure, the preparation method of described medicine is as follows:
[0054] Use 293T cells to add the packaging system at an appropriate cell density (80%); add 1 mL of the packaging system to each 10 cm dish: 10 μg AAV2 / 9 (purchased from Addgene Plasmid, catalog number #112865), 10 μg pAdDeltaF6 plasmid (purchased from Addgene Plasmid, Cat. No. #112867), 10 μg of the recombinant vector constructed in Example 2, add DMEM medium (no FBS and no double antibody) to 910 μl, add 90 μl PEI transfection reagent (total volume: 1 mL); collect the supernatant and its Cells; use iodixanol to concentrate and purify the virus by ultracentrifugation (after collecting the supernatant, use a concentration column (Merck UFC905096) at 4000rpm 4°C to concentrate all the supernatant by centrifugation to 10-15mL), and the obtained virus suspension is as described Drug; the titer of the virus suspension is 1×10 13 vg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com