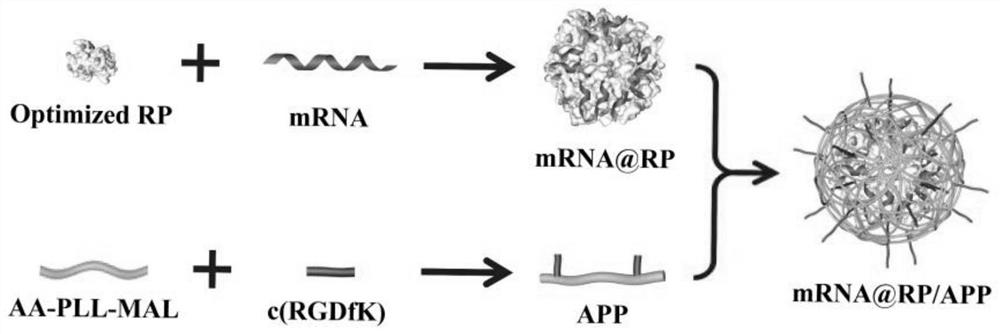
Nucleic acid drug delivery system based on recombinant ribosomal protein as well as preparation method and application of nucleic acid drug delivery system
A ribosomal protein and nucleic acid drug technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as limited protein expression efficiency, and achieve good transfection efficiency. Good application prospect and easy biodegradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] In one embodiment of the present invention, a method for preparing the above-mentioned recombinant ribosomal protein-based nucleic acid drug delivery system is provided, and the specific steps of the preparation method are as follows:
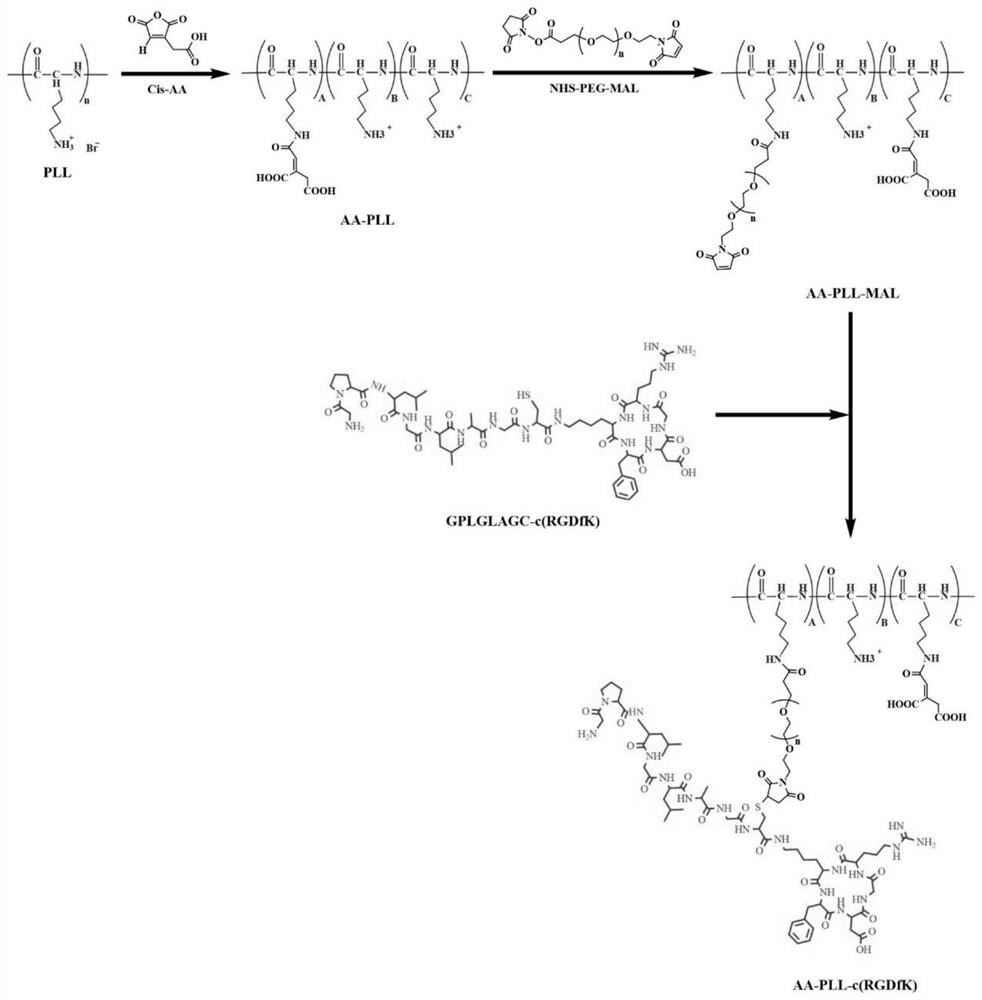
[0039](1) Preparation of poly-L-lysine derivative polymer (APP):
[0040] Poly-L-lysine and cis-aconitic anhydride were reacted for 6-10 hours at a pH of 9.0, the reaction solution was transferred to a dialysis bag, dialyzed in an aqueous solution with a pH of 8-9 for 22-26 hours, and then freeze-dried to obtain cis-aconitic anhydride. Poly-L-lysine AA-PLL grafted with aconitic acid;
[0041] React AA-PLL and NHS-PEG-MAL at pH 7.4 for 6-10 hours, dialyze and freeze-dry to obtain AA-PLL-MAL; graft RGD polypeptide on AA-PLL-MAL, and mix the two in an appropriate amount at pH React for 6-10 hours under the condition of 7.0 in an inert environment, and the product is obtained by dialysis and lyophilization to obtain maleimide-modified AA-PL...
Embodiment 1
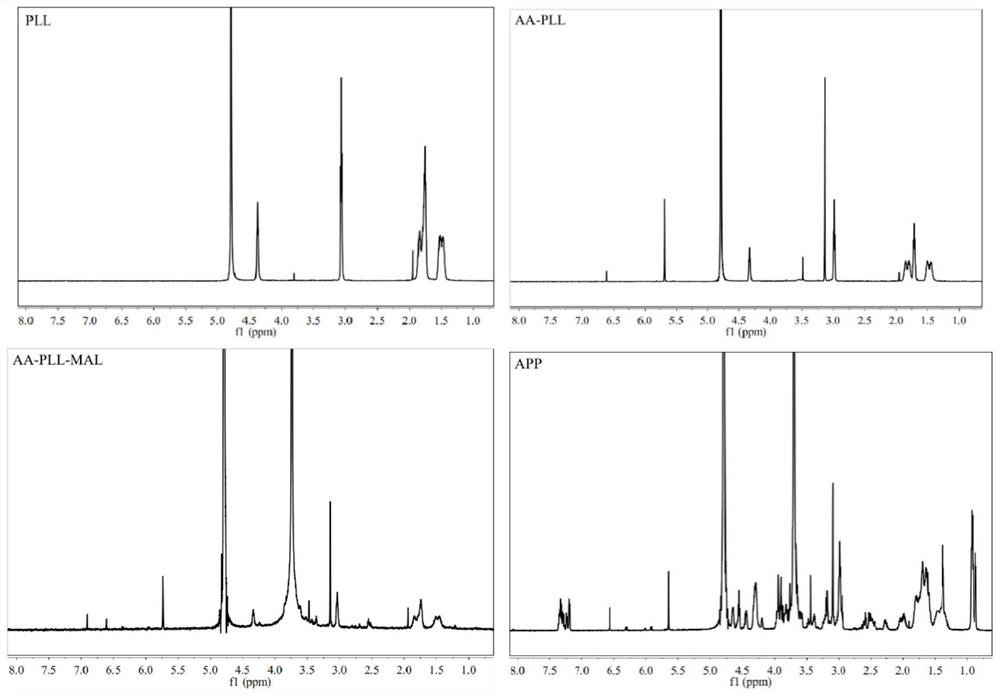
[0049] Synthesis of Multifunctional Poly-L-Lysine Derivatives (APP)
[0050] 1. Synthesis of poly-L-lysine-AA-PLL grafted with cis-aconitic acid
[0051] Accurately weigh 5.0mg of poly-L-lysine and dissolve in 5ml of PBS with pH 9.0, then add precisely weighed 4.2mg of cis-aconitic anhydride to dissolve, and dissolve with 1.0M NaOH to adjust the pH of the mixed solution to about 9.0 , reacted at room temperature for 8 hours under electromagnetic stirring, transferred the reaction mixture to a dialysis bag with a molecular weight cut-off of 3500Da after leak detection in advance, dialyzed in an aqueous solution with a pH of 8-9 adjusted by NaOH for 24 hours, and then freeze-dried. Whether the target product is synthesized.
[0052] 2. Synthesis of AA-PLL-AA-PP-MAL modified by maleimide
[0053] Accurately weigh 9.0 mg of the above product AA-PLL and dissolve it in 5 ml of PBS with pH 7.4, add 26.0 mg of NHS-PEG-MAL into the AA-PLL solution, and react at room temperature for 8...
Embodiment 2
[0057] Poly-L-lysine-derived polymer-modified ribosomal protein-enriched mRNA delivery system—preparation of mFluc@RP / APP nanocomplex
[0058] Mix a series of ribosomal proteins RPL28, RPL32, RPL27, RPS17, RPL22 for screening with luciferase mRNA (the mass ratio of ribosomal protein to mRNA is 1, 2, 4, 8, 16 respectively), pipette Gently pipette for 10s, and incubate at room temperature for 30 minutes to promote the full combination of cationic protein and mRNA to form mFluc@RP complex. After centrifugation and resuspension, add a certain amount of poly-L-lysine derivative polymer (APP: protein 40:1 mass ratio), blow gently with a pipette gun for 10 seconds, incubate at room temperature for 30 minutes, and centrifuge and resuspend to obtain the prepared mFluc@RP / APP nanocomplex, which is an mRNA delivery system based on cationic ribosomal protein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mwco | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com