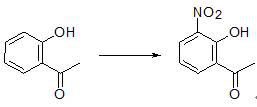
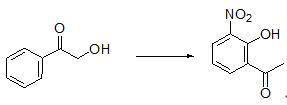
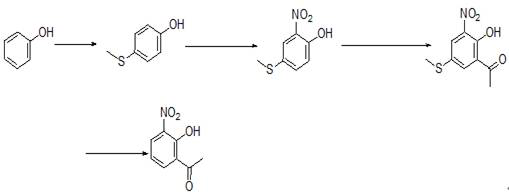
Preparation method of 2-hydroxy-3-nitroacetophenone
A technology of nitroacetophenone and hydroxyl, applied in the field of medicine, can solve the problems of little industrial significance, poor yield selectivity, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of 2-hydroxyl-3-nitroacetophenone, specifically comprising the following steps:
[0030] (1) Add 700ml of dichloromethane into the reaction flask, add 94.1g of phenol and 161.6g of N-methylmorpholine under stirring, slowly add 123.8g of chlorothiomethane dropwise at a temperature of 20°C~30°C, and stir after adding React for 10 hours, concentrate to dryness under reduced pressure, add 800ml of ethyl acetate and 300ml of water, stir and separate the liquids, dry the organic phase with anhydrous sodium sulfate, decolorize with activated carbon under reflux, filter, concentrate the filtrate to dryness, and wash with 250ml of toluene Recrystallized, filtered and dried to obtain 120.1 g of intermediate Ⅰ, a reddish solid, with a yield of 85.7%.
[0031] (2) Add 1100ml of ethanol to the reaction flask, add 120.1g of intermediate I under stirring, add 266.1g of ferric nitrate in batches under temperature control at 50°C~60°C, and react for 14 hours after a...
Embodiment 2
[0035] A preparation method of 2-hydroxyl-3-nitroacetophenone, specifically comprising the following steps:
[0036] (1) Add 900ml of tetrahydrofuran into the reaction flask, add 94.1g of phenol and 90.6g of copper diacetate under stirring, slowly add 103.6g of dimethyl disulfide dropwise under the condition of keeping warm at 50°C~60°C, and stir for 24 hours after completion of the reaction , concentrated to dryness under reduced pressure, added 800ml of ethyl acetate and 300ml of water, stirred and left to separate the liquids, the organic phase was dried with anhydrous sodium sulfate and then decolorized with activated carbon under reflux, filtered, the filtrate was concentrated to dryness, recrystallized with 250ml of toluene, Filtration and drying yielded 110.1 g of intermediate I as a reddish solid with a yield of 78.5%.
[0037] (2) Add 800ml of acetic acid into the reaction flask, add 110.1g of intermediate I under stirring, add 86.1g of nitric acid with a mass concent...
Embodiment 3
[0041] (1) Add 1100ml of chloroform into the reaction flask, add 94.1g of phenol and 146.8g of anhydrous aluminum trichloride under stirring, slowly add 141.3g of dimethyl disulfide dropwise under the condition of keeping warm at 40°C~50°C, add After stirring and reacting for 6 hours, concentrate under reduced pressure to dryness, add 800ml of ethyl acetate and 300ml of water, stand for liquid separation after stirring, stand for liquid separation after stirring, dry the organic phase with anhydrous sodium sulfate, decolorize with activated carbon under reflux, and filter , the filtrate was concentrated to dryness, recrystallized with 250 ml of toluene, filtered and dried to obtain 103.9 g of intermediate I, an off-white solid, with a yield of 74.1%.
[0042](2) Add 600ml of acetonitrile into the reaction flask, add 103.9g of intermediate I under stirring, add 133.9g of tert-butyl nitrite dropwise under temperature control at 30°C~40°C, react for 48 hours after addition, and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com