Method for preparing titanium niobium oxide
A technology of titanium niobium oxide and niobium source, applied in niobium compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of difficulty in taking into account the performance and production cost of titanium niobium oxide, and achieves increased lithium ion transmission performance, improved Effect of particle morphology, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Titanium niobium oxide TiNb 6 o 17 preparation of
[0051] Ethylene glycol is used as a solvent, P123 is used as a surfactant; niobium oxalate is used as a niobium source, and titanium isopropoxide is used as a titanium source;
[0052] S1, add 20ml of ethylene glycol to the beaker, then add 0.1g of P123, 6.456g of niobium oxalate and 0.505ml of titanium isopropoxide into the beaker;
[0053] S2, heated to 160°C, concentrated to form a gel;
[0054] S3, calcining the gel obtained in step S2 at 800° C. for 5 hours in an air atmosphere to obtain a powder, and ball milling and drying the powder to obtain titanium niobium oxide.
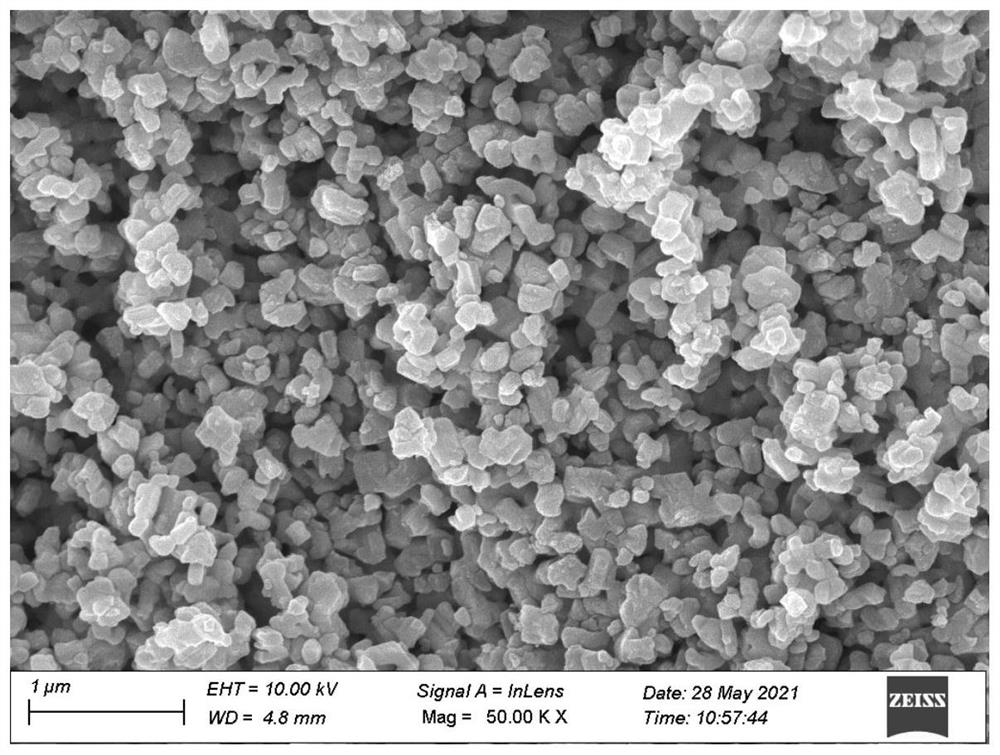
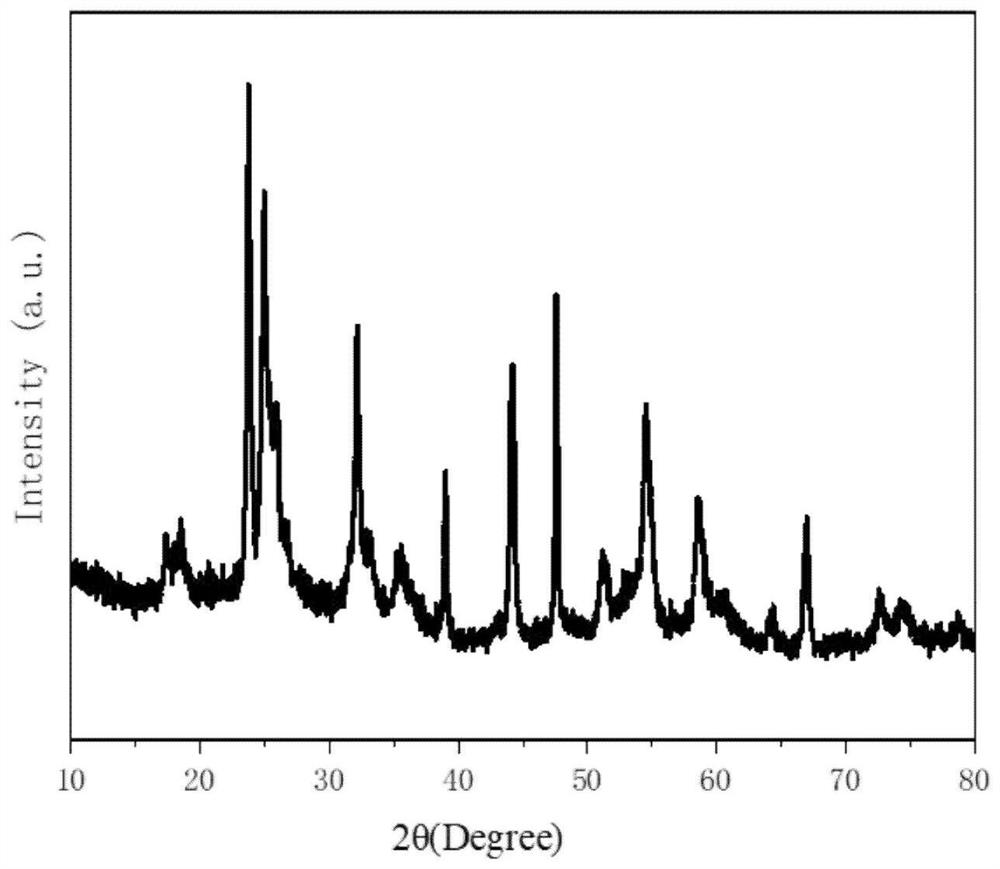
[0055] The SEM of the titanium niobium oxide is as figure 1 Shown, XRD test result is as follows figure 2 shown.
[0056] The performance of the obtained titanium niobium oxide is tested, the test method is: the obtained TiNb 6 o 17 Mix it with conductive carbon black and PVDF, add NMP as a solvent and coat it on copper foil to make an el...
Embodiment 2
[0058] Titanium niobium oxide TiNb doped with carbon and oxygen vacancies 6 o 17 preparation of
[0059] Ethylene glycol is used as a solvent, P123 is used as a surfactant; niobium oxalate is used as a niobium source, and tetrabutyl titanate is used as a titanium source;
[0060] S1, add 20ml of ethylene glycol to the beaker, then add 0.2g of P123, 12.912g of niobium oxalate and 1.36ml of tetrabutyl titanate into the beaker;
[0061] S2, heated to 150°C, concentrated to form a gel;
[0062] S3, calcining the gel obtained in step S2 at 500°C for 2 hours in an air atmosphere; then sintering at 900°C for 3 hours in an argon atmosphere to obtain a powder, and ball milling and drying the powder to obtain titanium niobium oxide doped with carbon and oxygen vacancies .
[0063] The SEM of the titanium niobium oxide is as Figure 4 As shown, the TEM test results are as follows Figure 5 As shown, the performance of the obtained titanium-niobium oxide was tested, and the test met...
Embodiment 3
[0066] Titanium niobium oxide TiNb 2 o 7 preparation of
[0067] 1,2-propanediol is used as solvent, P123 is used as surfactant; ammonium niobium oxalate is used as niobium source, and tetrabutyl titanate is used as titanium source.
[0068] S1, add 20ml of 1,2-propanediol to the beaker, then add 0.1g of P123, 7.5g of ammonium niobium oxalate and 3.4ml of tetrabutyl titanate into the beaker;
[0069] S2, heated to 180°C, concentrated to form a gel;
[0070] S3, calcining the gel obtained in step S2 at 900°C for 3 hours in an air atmosphere to obtain a powder, which is ball milled and dried to obtain TiNb 2 o 7 .
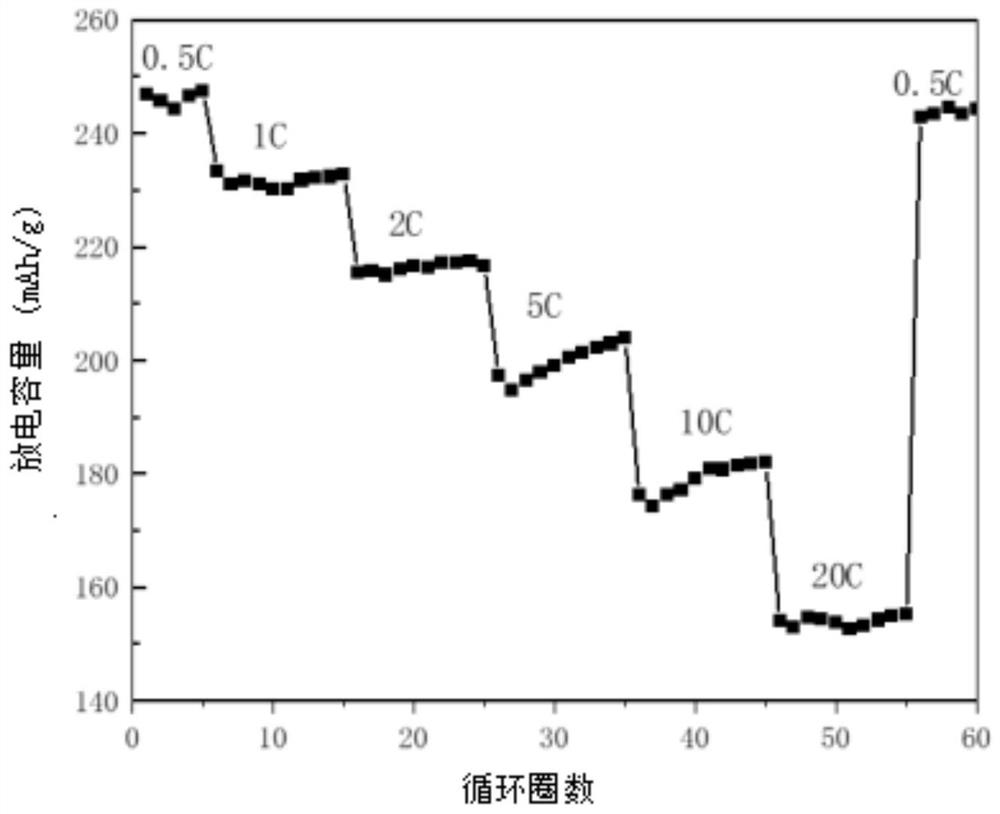
[0071] The SEM of the titanium niobium oxide is as Figure 7 Shown, XRD test result is as follows Figure 8 As shown, the rate performance as Figure 9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com