Synthesis method of prothioconazole
A synthesis method and technology of prothioconazole, applied in the field of organic synthesis, can solve the problems of large amount of oxidant, many impurities in the final product, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
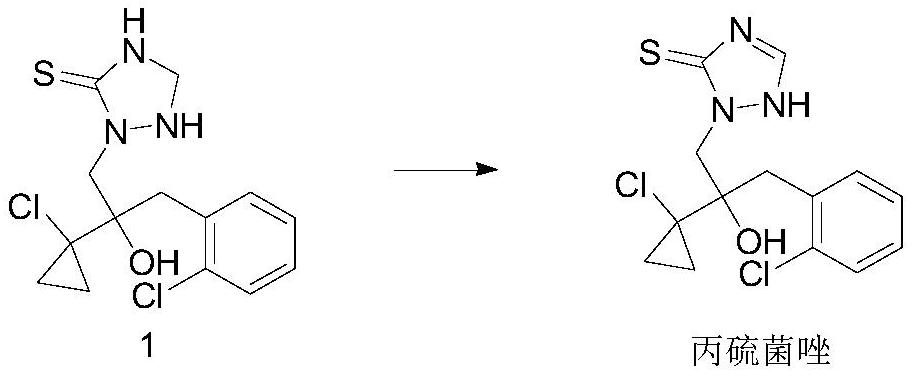
[0035] The synthetic method of described kind of high-yield prothioconazole and aftertreatment thereof, comprises the following steps:
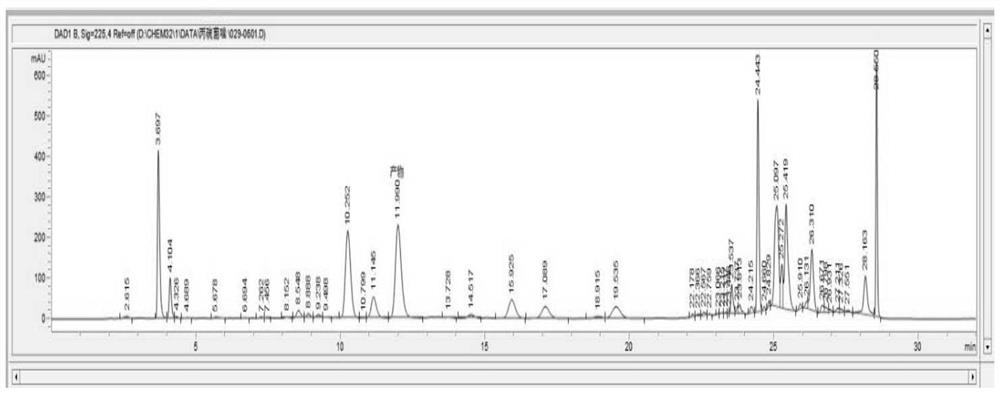
[0036] 1) Add compound 1 to the solvent, add a certain amount of titanium-silicon molecular sieve, add a certain amount of hydrogen peroxide dropwise at a certain temperature, keep warm for a period of time after the addition is completed, and monitor the raw material within 1% by liquid chromatography; Filtrate, recover the catalyst titanium-silicon molecular sieve and apply mechanically, the filtrate is allowed to stand and separate into layers to separate the water phase, and the organic phase is precipitated to obtain the product prothioconazole;
[0037] Apply titanium silicate molecular sieve steps:
[0038] 2) Add compound 1 into the solvent, add recovered titanium-silicon molecular sieve, add a certain amount of hydrogen peroxide dropwise under certain temperature conditions, keep warm for a period of time after the addition is comple...
Embodiment 1
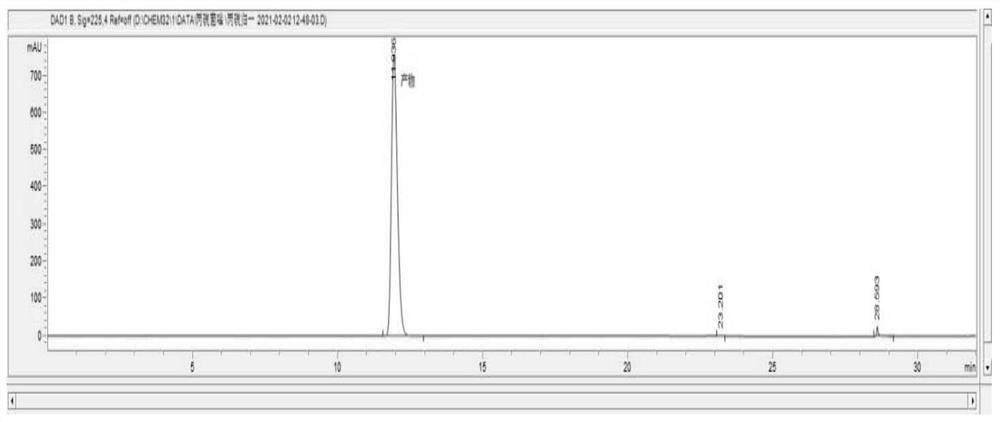
[0053] Compound 1,2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1,2,4-triazolidine-5-thione -1-yl)-propane (69.2g, 0.2mol) was added to 210g toluene, 2.07g of titanium silicon molecular sieve was added, the temperature was raised to 40°C, 10% hydrogen peroxide (68.0g, 0.2mmol) was added dropwise, and the temperature of the reaction solution was controlled to 40- 45°C, after the dropwise addition is completed, keep at 40°C for 3 hours. After the reaction, the temperature was lowered to 25°C, the titanium silicon molecular sieve was recovered by filtration, the filtrate was allowed to stand for stratification, and the organic phase was desolvated to obtain a white solid, namely 68.0 g of prothioconazole, with a content of 98.4% and a yield of 97.3%.
[0054] The nuclear magnetic data of gained product is: 1 HNMR (600MHz, DMSO-d 6 ) δ (ppm) 13.67 (lH, s), 8.43 (lH, s), 7.53-7.55 (lH, m), 7.37-7.39 (lH, m), 7.21-7.26 (2H, m), 5.06 (lH, s), 4.48(2H, s), 3.34(lH,...
Embodiment 2
[0057] Compound 1,2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1,2,4-triazolidine-5-thione -1-yl)-propane (69.2g, 0.2mol) was added to 180g dichloroethane, 2.07g titanium silicon molecular sieve was added, the temperature was raised to 40°C, 10% hydrogen peroxide (68.0g, 0.2mmol) was added dropwise, and the reaction solution was controlled The temperature is 40-45°C, and the temperature is kept at 40°C for 3 hours after the dropwise addition is completed. After the reaction, the temperature was lowered to 25°C, the titanium silicon molecular sieve was recovered by filtration, the filtrate was allowed to stand for stratification, and the organic phase was desolvated to obtain a white solid, which was prothioconazole 67.9g, with a content of 98.4%, and a yield of 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com