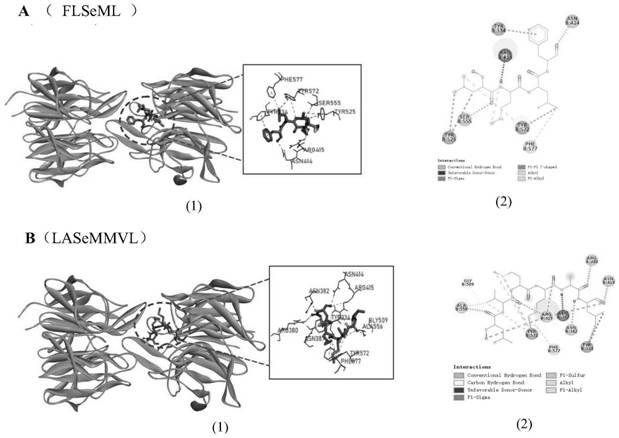
Selenium-rich peptide with high antioxidant activity and application thereof
An oxidation-active, selenium-enriched technology, applied in the field of anti-oxidation, can solve problems such as research needs to be further deepened, and achieve the effect of high selenium content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
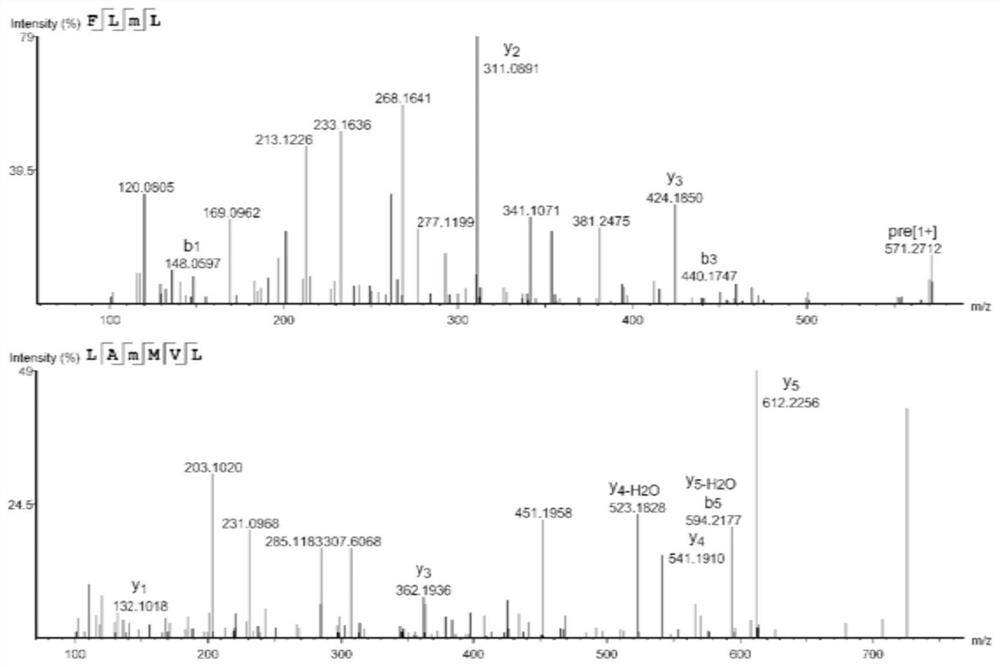
[0041] The separation and purification of embodiment 1 selenium-enriched peptide
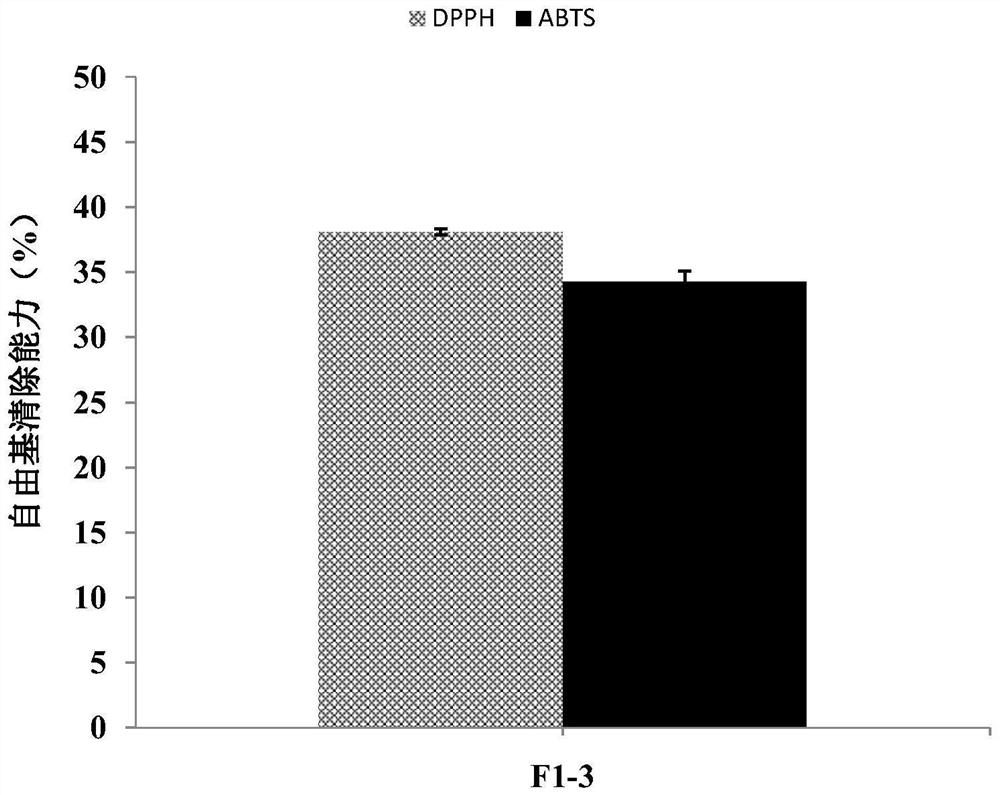
[0042] The DPPH and ABTS free radical scavenging rate and selenium content of the F1-3 fraction separated from the selenium-enriched Moringa oleifera seed protein hydrolyzate were determined. The test process and results are as follows:
[0043] (1) DPPH free radical scavenging rate
[0044] Testing process:
[0045] Add 100 μL of sample solution to 100 μL of 0.15 mM DPPH solution. The mixture was then left at room temperature in the dark for 30 minutes. Measure the absorbance of the 96-well plate at 517 nm using a microplate reader. The calculation formula of DPPH free radical scavenging rate is:
[0046] DPPH free radical scavenging rate (%)=[1-(A 样品 -A 空白 ) / A 对照 ]×100 (1)
[0047] A 样品 : Absorbance of adding 100 μL sample solution to 100 μL DPPH solution;
[0048] A 空白 : Absorbance of adding 100 μL ethanol to 100 μL sample solution;
[0049] A 对照 : Absorbance of 100 μL DPPH soluti...
Embodiment 3
[0072] Antioxidant activity assay of embodiment 3 selenium-enriched peptides
[0073] The antioxidant activities of the two selenium-rich peptides obtained in Example 2 were determined.
[0074] (1) Toxic effect (MTT) detection of selenium-enriched peptides on HepG2 cells
[0075] HepG2 cell culture: 10% FBS mixed with DMEM medium, the cells were placed in 5% CO 2 Cultured in a cell culture incubator. When the HepG2 cells adhered to the wall and fused into a dense monolayer and the cell coverage reached more than 80%, they were digested and passaged with 0.25% trypsin solution or carried out to the next step.
[0076] Toxicity detection of selenium-rich peptides on HepG2 cells: 1×10 5 cells / mL of HepG2 cells were inoculated in 96-well plates (6 replicate wells for each group), set up control group and test group, added 100 μL cell suspension to the control group and test group, cultured for 24 hours, and the cells adhered to the wall, and the control group was added Add 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com