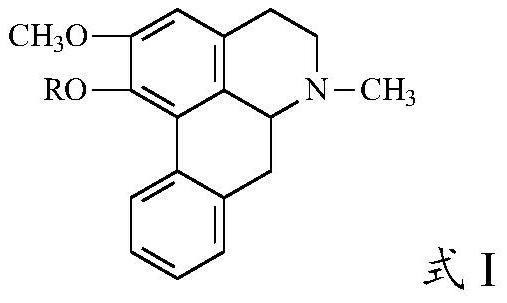
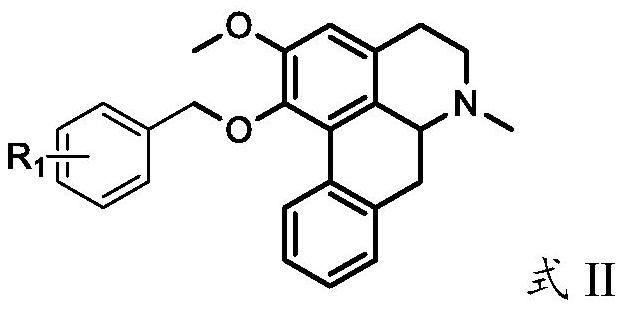
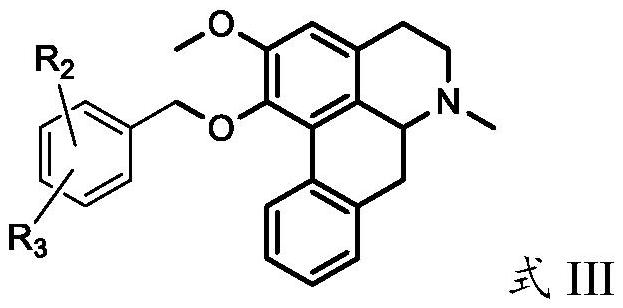
Nuciferine derivative as well as preparation method and application thereof
A lotus leaf alkaloid derivative, the technology of lotus leaf alkaloid, which is applied in the field of medicine, can solve the problems of weak lipid-lowering activity and the like, and achieves the improvement of adsorption capacity, increased hypoglycemic and lipid-lowering activities, and good hypoglycemic and lipid-lowering activities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1 Synthesis of 1-O-benzylnuciline
[0228] Accurately weigh 2.81g (10mmol) of 1-O-desmethylnuciferine, dissolve it in 50mL of dichloromethane solvent and place it in a 250mL three-necked flask filled with nitrogen, add 0.29g (about 12mmol) of sodium hydride, and mechanically Stir and mix for 2.0 hours, place in a cold trap, add 1.27g (about 10mmol) benzyl chloride dissolved in 30mL of dichloromethane, and control the temperature at -20~-10°C to react until 1-O-desmethyl nuciferine reacts Complete, add 60mL of deionized ice water after the reaction, separate the organic phase, use dichloromethane for the water phase 3 times (for example, 50mL×3), combine the organic phase, evaporate the organic phase to obtain 2.21g of light yellow powder product, TLC Tracking the reaction and the separation and purification process of the product, the melting point of the product is 103.5-105.2 ° C, after 1 H NMR, 13 C NMR and HR-MS analysis, determined to be 1-O-benzylnucif...
Embodiment 2
[0229] Example 2 Synthesis of 1-O-O-Fluorobenzyl Nuciferine
[0230] Accurately weigh 2.81g (10mmol) of 1-O-desmethylnuciferine, dissolve it in 30mL of DMF solvent and place it in a 250mL three-necked flask filled with nitrogen, add 0.56g (about 10mmol) of potassium hydroxide, and mechanically stir at room temperature Mix for 2.0 hours, add 2.89g (about 20mmol) o-fluorobenzyl chloride dissolved in 50mL DMF, react at 30-40°C until the reaction of 1-O-desmethylnuciferine is complete, and use 60mL deionized ice Wash 3-5 times with water, concentrate the organic phase to about 10 mL by rotary evaporation, separate and purify through a neutral alumina column (eluent v / v: dichloromethane / methanol=200 / 1-100 / 1), and collect product fractions , The solvent was distilled off under reduced pressure to obtain the product, TLC traced the separation and purification process of the reaction and the product, and obtained 2.44 g of a light green powdery product. The melting point of the pro...
Embodiment 3
[0231] Example 3 Synthesis of 1-O-m-fluorobenzylnuciferine
[0232] Accurately weigh 2.81g (10mmol) of 1-O-desmethylnuciferine, dissolve it in 30mL DMF solvent and place it in a 250mL three-necked flask filled with nitrogen, add 3.41g (about 40mmol) of piperidine, and mix mechanically at room temperature 2.0h, add 2.17g (about 15mmol) m-fluorobenzyl chloride dissolved in 50mL DMF, react at 90-100°C until the reaction of 1-O-desmethylnuciferine is complete, wash with 60mL deionized ice water after the reaction 3-5 times, the organic phase was concentrated by rotary evaporation to about 20mL, crystallized at room temperature for 4-6h, filtered with suction, and the solid was dried at 60°C for 4-6h to obtain the product. TLC traced the separation and purification process of the reaction and the product to obtain 2.03 g of light green powdery product, the melting point of the product is 102.7-104.5 ° C, after 1 H NMR, 13 According to C NMR and HR-MS analysis, it was determine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com