Preparation method and application of gel for in-situ delivery of nano-micelles
A nanomicelle and gel technology, applied in the field of biomedical materials, can solve the problem of limited accumulation of nanomicelles, and achieve the effects of improving bioavailability, avoiding systemic toxicity, and enhancing chemotherapy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
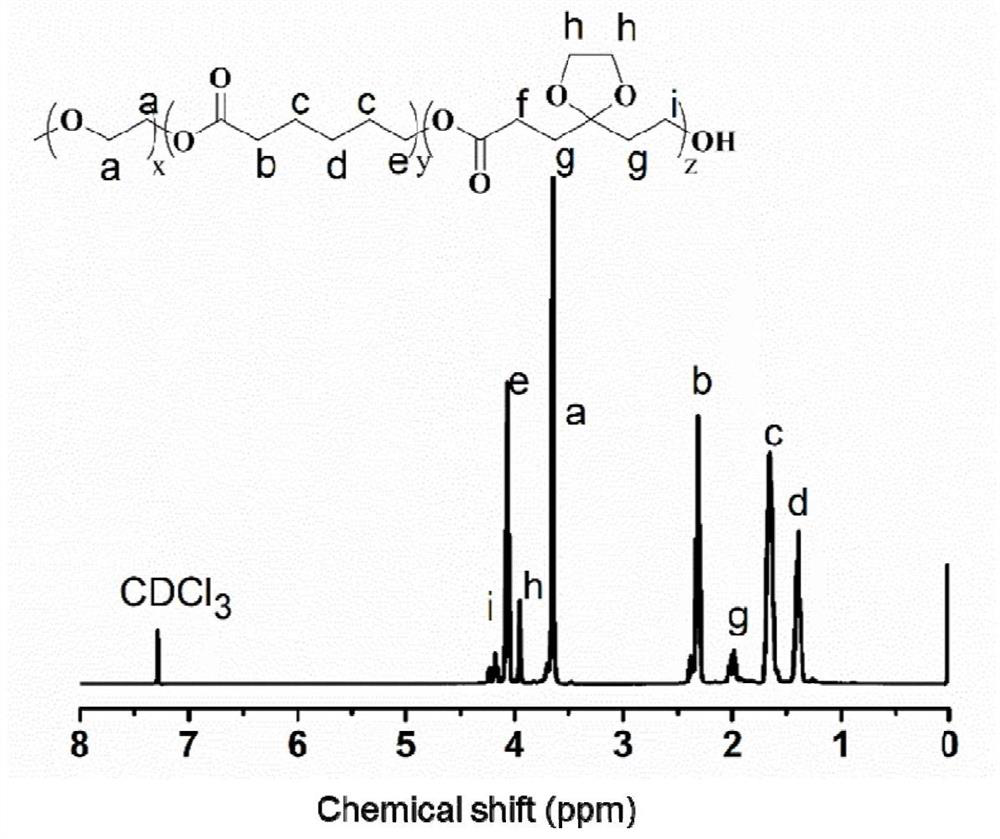
[0039] Example 1, the synthesis and characterization of the polymer poly(5-ethylene glycol ketal-ε-caprolactone-ε-caprolactone)-polyethylene glycol monomethyl ether.
[0040] Weigh 5.0 g of polyethylene glycol monomethyl ether (molecular weight: 2000 Da) into a 25 mL dry glass reactor, dry in vacuum at 60°C for 1 hour, add 2.74 g of monomer 5-ethylene glycol under nitrogen protection Ketal-ε-caprolactone and 7.26g of ε-caprolactone, then add 0.1mL stannous octoate, degas and seal under reduced pressure. After stirring at 130°C for 6 hours, it was dissolved in dichloromethane, and then added dropwise to an excess of cold ether for precipitation. After the precipitate was filtered, it was vacuum-dried at room temperature to obtain the polymer poly(5-ethylene glycol ketal) -ε-caprolactone-ε-caprolactone)-polyethylene glycol monomethyl ether.
[0041] synthetic polymer 1 The H NMR spectrum is attached figure 1 As shown, the structure of the polymer and the corresponding NMR pea...
Embodiment 2
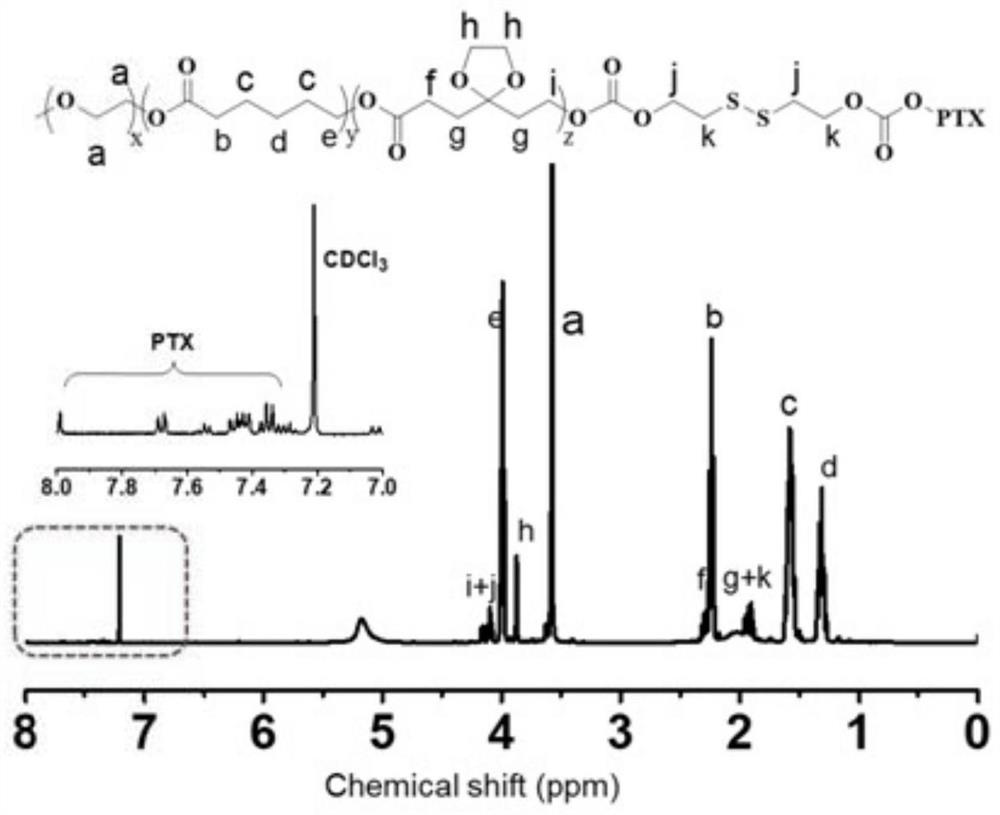
[0042] Example 2, the synthesis and characterization of the polymer poly(5-ethylene glycol ketal-ε-caprolactone-ε-caprolactone)-polyethylene glycol monomethyl ether with paclitaxel molecules bonded by disulfide bonds.
[0043] Weigh 1.0 g of poly(5-ethylene glycol ketal-ε-caprolactone-ε-caprolactone)-polyethylene glycol monomethyl ether synthesized in Example 1, and dissolve it in 10 mL of dichloromethane , add dropwise a dichloromethane solution (5 mL) of 0.05 g of triphosgene in an ice-water bath, and after incubation for 30 minutes, add a solution of 0.052 g of 2-hydroxyethyl disulfide in dichloromethane (5 mL) . After reacting at room temperature for 24 hours, it was added dropwise into pre-cooled diethyl ether to precipitate, and the product was obtained after filtration. Further, the product was dissolved in 10 mL of tetrahydrofuran, dialyzed in water with a dialysis bag with a molecular weight cut-off of 3.5 KDa, and the product was freeze-dried after dialysis.
[004...
Embodiment 3
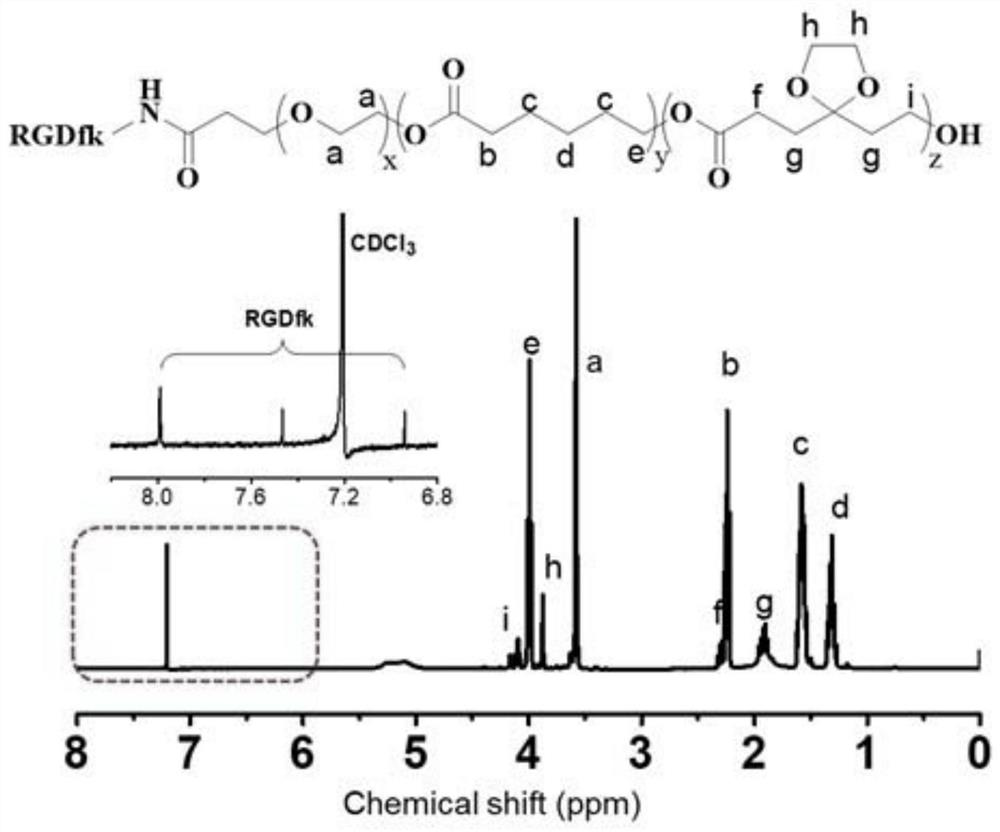
[0045] Example 3, synthesis and characterization of modified RGD cyclic peptide-polyethylene glycol-poly(5-ethylene glycol ketal-ε-caprolactone-ε-caprolactone).
[0046] The polyethylene glycol monomethyl ether in embodiment 1 is replaced by succinimide ester-polyethylene glycol (molecular weight is 2000kDa), all the other steps are identical with embodiment, synthetic succinimide ester-polyethylene glycol- Poly(5-ethylene glycol ketal-ε-caprolactone-ε-caprolactone); take 0.1 of the above polymer, dissolve it in 5 mL of PBS, add 0.015 g of cyclic RGD polypeptide, and incubate for another 24 hours. Then the target product was obtained after purification by dialysis.
[0047] The combination of the prepared products and 1 The H NMR spectrum is attached image 3 shown. The NMR peaks representing the cyclic RGD were observed, proving that the cyclic RGD was successfully indirect on the polymer chain segment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com