Silibinin nano suspension, lyophilized preparation thereof and preparation method of lyophilized preparation
A technology of silibinin and stabilizer, which is applied in the field of silibinin pharmaceutical preparations, can solve the problems of low bioavailability, limited application of silybin, poor water solubility, etc., achieve high drug content, improve chemical and physical Effect of stability and small particle size change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 10 mg of silibinin bulk drug (Sy), add 1 mL of absolute ethanol for ultrasonic dissolution to obtain a silibinin absolute ethanol solution; weigh 1.00 mg of stabilizer (soluplus: SDS=1:5) and dissolve in 7 mL of pure water In the process, the stabilizer solution was obtained, and the silibinin anhydrous ethanol solution was added dropwise to the stabilizer solution under the action of a cell ultrasonic pulverizer (65w ultrasonic for 31min) to make the dispersion uniform, and the obtained suspension was spun on a rotary evaporator. Steam until there is no alcohol smell to obtain a silibinin nanosuspension. 5% mannitol was added to the silibinin nano suspension, and after lyophilization for 48 hours, the silibinin nano freeze-dried powder was obtained.
Embodiment 2
[0023] Weigh 10 mg of silibinin API (Sy), add 1 mL of absolute ethanol for ultrasonic dissolution to obtain silibinin absolute ethanol solution; weigh 2.00 mg of stabilizer (soluplus: SDS=1:5) and dissolve in 10 mL of pure water In the process, the stabilizer solution was obtained, and the silibinin anhydrous ethanol solution was added dropwise to the stabilizer solution under the action of a cell ultrasonic pulverizer (65w ultrasonic for 31min) to make the dispersion uniform, and the obtained suspension was spun on a rotary evaporator. Steam until there is no alcohol smell to obtain a silibinin nanosuspension. 5% mannitol was added to the silibinin nano suspension, and after lyophilization for 48 hours, the silibinin nano freeze-dried powder was obtained.
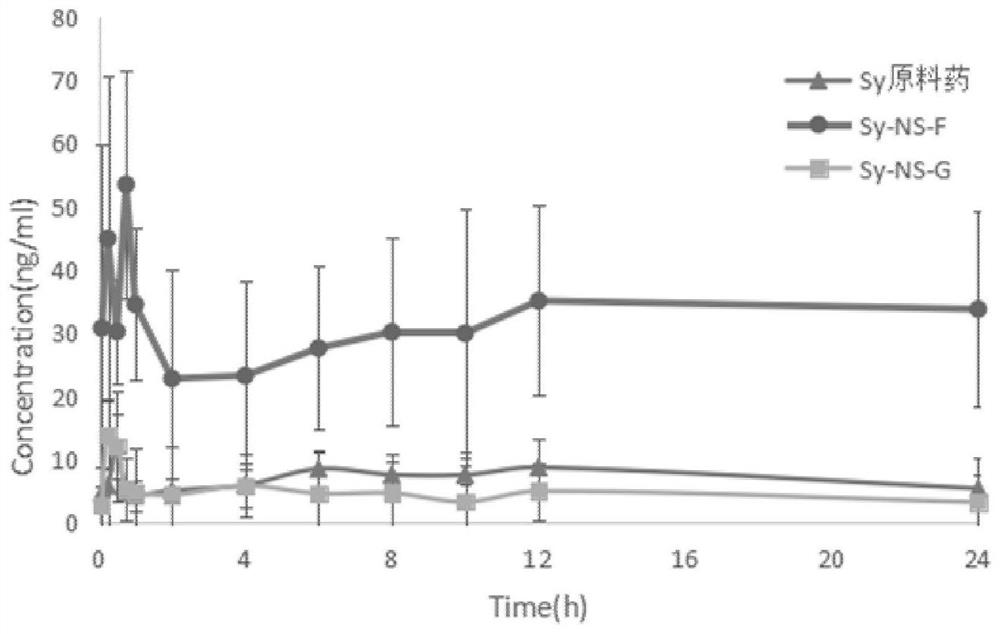
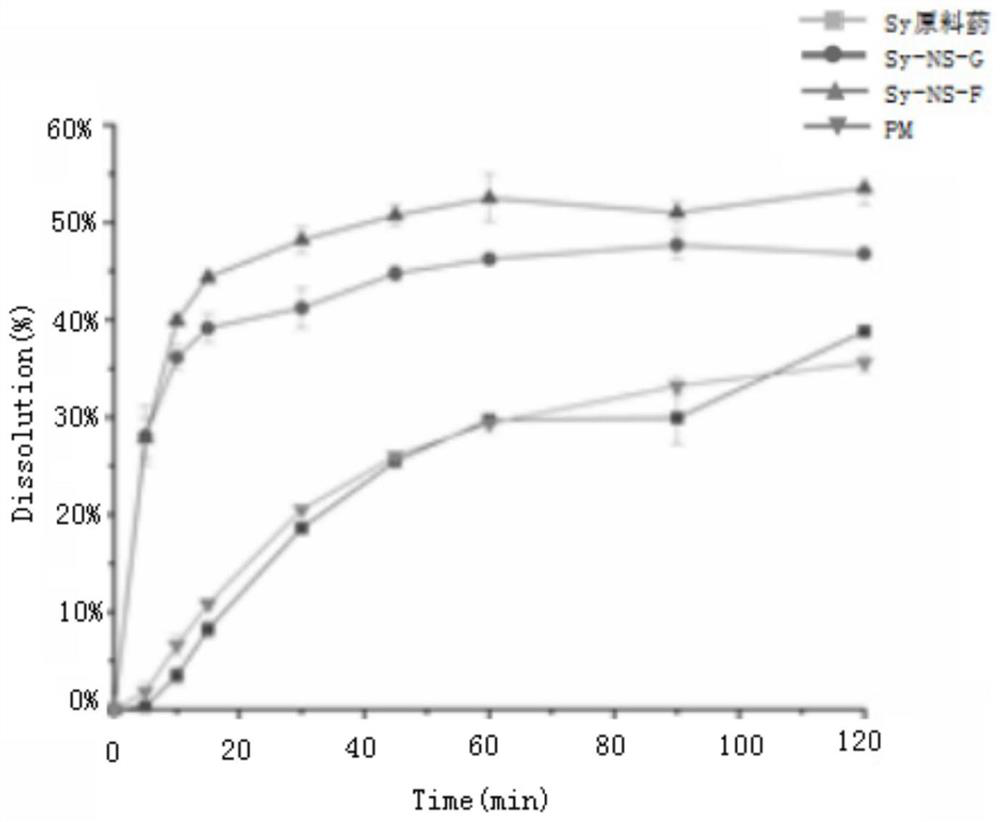
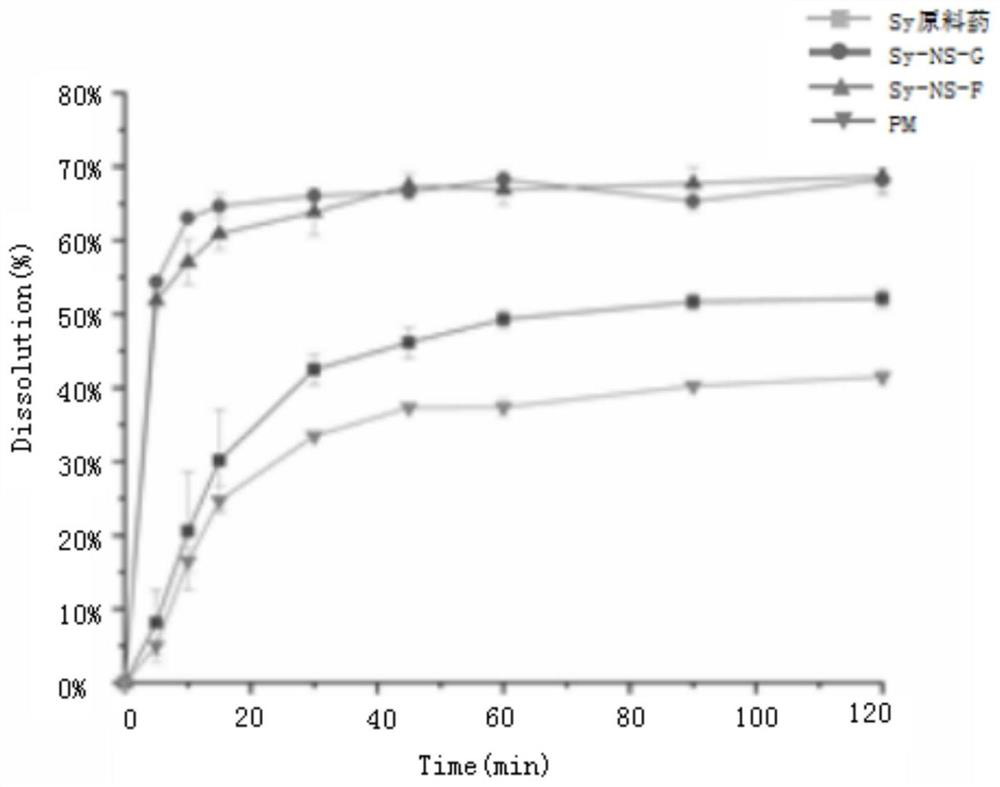
[0024] The silibinin nanosuspension and silibinin nano freeze-dried powder prepared in Examples 1-2 were respectively taken to measure particle size and PDI. The results are shown in the table below.
[0025] Table 1 Sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com