Preparation method of high-purity lithium hexafluorophosphate
A lithium hexafluorophosphate, high-purity technology, applied in the directions of lithium hexafluorophosphate, chemical instruments and methods, phosphorus compounds, etc., to achieve the effect of good purification effect, less waste discharge, and safe production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
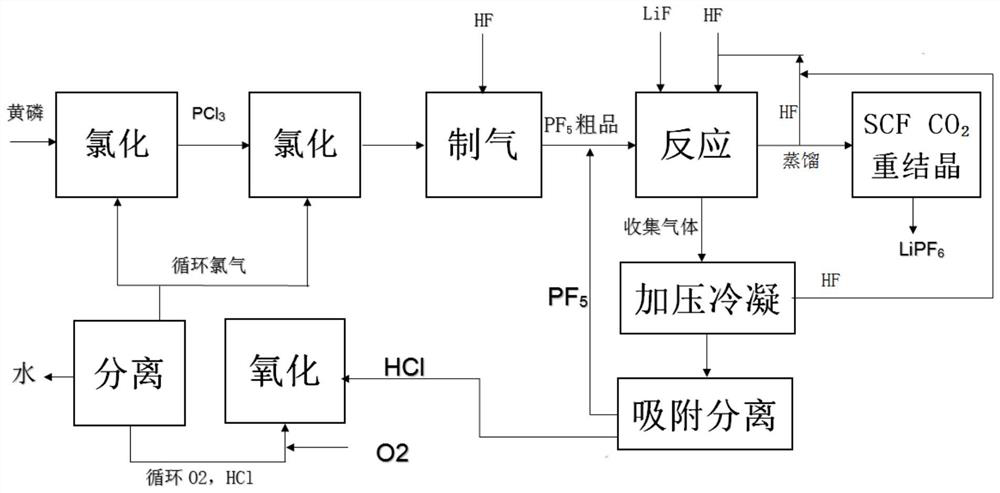
[0034] like figure 1 As shown, a preparation method of high-purity lithium hexafluorophosphate, comprising the following steps:
[0035] 1) reacting phosphorus pentachloride with anhydrous hydrofluoric acid or hydrogen fluoride gas to prepare phosphorus pentafluoride; the phosphorus pentachloride is prepared by reacting phosphorus trichloride and chlorine gas; the preparation of the phosphorus pentachloride The raw material phosphorus trichloride is prepared by the reaction of yellow phosphorus and chlorine;
[0036] 2) LiF is dissolved in the anhydrous HF solvent, and then pass into step 1) gained phosphorus pentafluoride, phosphorus pentafluoride and LiF react to obtain the anhydrous HF solution of lithium hexafluorophosphate, the mol ratio of phosphorus pentafluoride and LiF About 1.1~1.2:1;
[0037] 3) distilling the anhydrous HF solution of the obtained lithium hexafluorophosphate to remove hydrofluoric acid, and the distillation temperature is 30~50 ℃ to obtain solid l...
Embodiment 2
[0047] A preparation method of high-purity lithium hexafluorophosphate, comprising the following steps:
[0048] 1) reacting phosphorus pentachloride with anhydrous hydrofluoric acid or hydrogen fluoride gas to prepare phosphorus pentafluoride; the phosphorus pentachloride is prepared by reacting phosphorus trichloride and chlorine gas; the preparation of the phosphorus pentachloride The raw material phosphorus trichloride is prepared by the reaction of yellow phosphorus and chlorine;
[0049] 2) LiF is dissolved in the anhydrous HF solvent, and then pass into step 1) gained phosphorus pentafluoride, phosphorus pentafluoride and LiF react to obtain the anhydrous HF solution of lithium hexafluorophosphate, the mol ratio of phosphorus pentafluoride and LiF About 1.1~1.2:1;
[0050] 3) distilling the anhydrous HF solution of the obtained lithium hexafluorophosphate to remove hydrofluoric acid, and the distillation temperature is 30~50 ℃ to obtain solid lithium hexafluorophosphat...
Embodiment 3
[0056] A preparation method of high-purity lithium hexafluorophosphate, comprising the following steps:
[0057] 1) reacting phosphorus pentachloride with anhydrous hydrofluoric acid or hydrogen fluoride gas to prepare phosphorus pentafluoride; the phosphorus pentachloride is prepared by reacting phosphorus trichloride and chlorine gas; the preparation of the phosphorus pentachloride The raw material phosphorus trichloride is prepared by the reaction of yellow phosphorus and chlorine;
[0058] 2) LiF is dissolved in the anhydrous HF solvent, and then pass into step 1) gained phosphorus pentafluoride, phosphorus pentafluoride and LiF react to obtain the anhydrous HF solution of lithium hexafluorophosphate, the mol ratio of phosphorus pentafluoride and LiF About 1.1~1.2:1;
[0059] 3) distilling the anhydrous HF solution of the obtained lithium hexafluorophosphate to remove hydrofluoric acid, and the distillation temperature is 30~50 ℃ to obtain solid lithium hexafluorophosphat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com