Lipid nanoparticles and use thereof in nucleic acid delivery
A technology of lipid nanoparticles and lipids, which can be used in medical preparations of non-active ingredients, other methods of inserting foreign genetic materials, capsule delivery, etc., and can solve problems such as limiting LNP transfection ability and pDNA immobilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
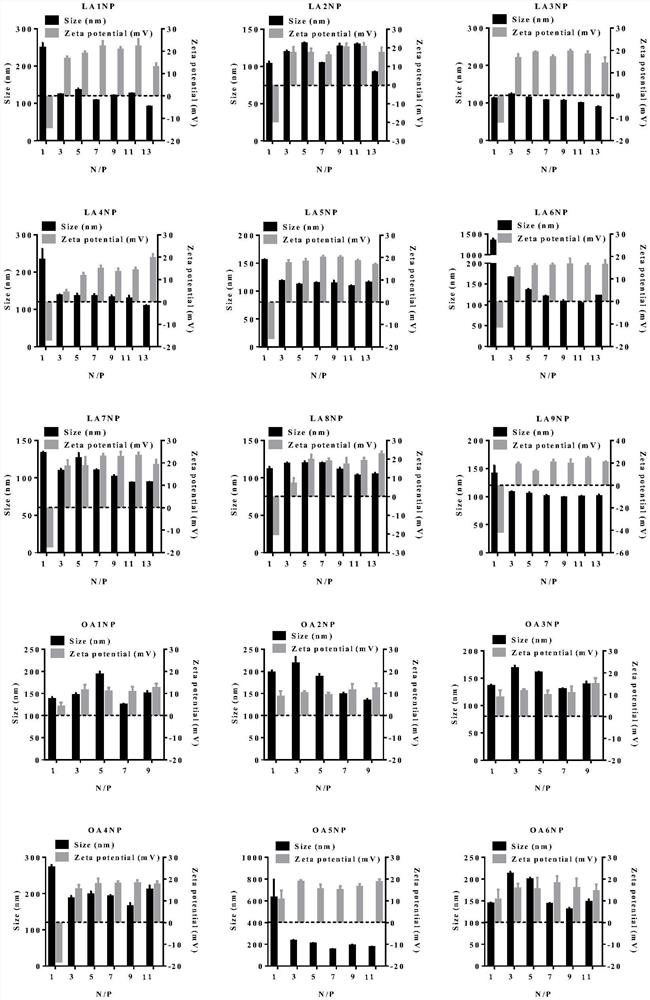
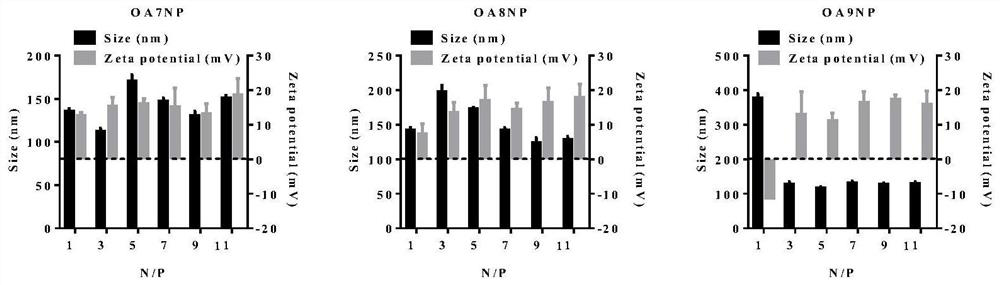
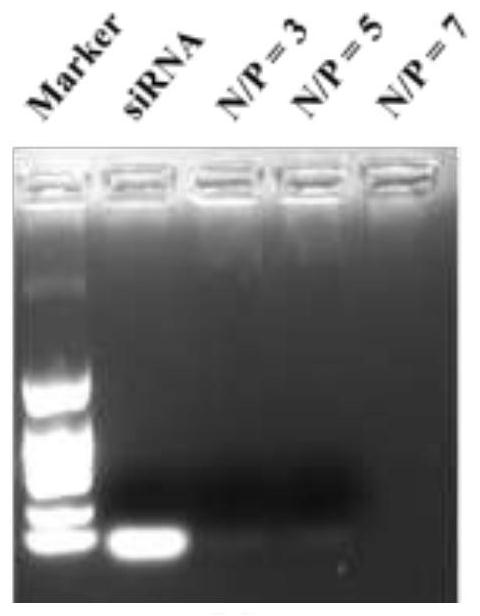
Image
Examples
Embodiment 1
[0082] Preparation of glutamic acid dilinolenic acid ester (LA 2 -NH 2 ), the chemical structure is as follows:
[0083]
[0084] L-glutamic acid (3.00g, 20.60mmol), p-toluenesulfonic acid (4.30g, 22.66mmol) and anhydrous toluene (150mL) were added to the 250mL reaction flask, then the reaction solution was heated to 140°C, and the reaction was refluxed for 3h . After the heating was stopped, the mixed solution was cooled to room temperature, linolenic alcohol (11.53 g, 43.25 mmol) was added in batches, and the reaction was carried out at 140° C. overnight. After the reaction, the toluene was removed by rotary evaporation to obtain a dark brown oil. The crude product was dissolved in dichloromethane, washed twice with an appropriate amount of water, twice with saturated aqueous sodium bicarbonate solution, once with saturated brine, dried over anhydrous sodium sulfate, and concentrated by suction filtration to obtain a dark yellow oil. Purified by column chromatography ...
Embodiment 2
[0086] Preparation of carboxylated glutamic acid bislinolenol ester (LA 2 -COOH), the chemical structural formula is as follows:
[0087]
[0088] will LA 2 -NH 2 (1.21 g, 1.88 mmol) was dissolved in dichloromethane, triethylamine (1.04 mL, 7.51 mmol) and succinic anhydride (188 mg, 1.88 mmol) were successively added thereto under stirring, and the reaction was carried out at room temperature overnight. After the reaction, the solvent was removed by rotary evaporation to obtain a light yellow oily crude product, which was purified by column chromatography (dichloromethane:methanol=50:1) to obtain 1.20 g of a light yellow oily product, yield: 85.7%. 1 H NMR (300MHz, CDCl 3 ):δ(ppm)7.53(d,J=8.7Hz,1H,CONHCH),5.43-5.26(m,8H,CHCH),4.51(d,J=5.4Hz,1H,CONHCH),4.11(t,J =6.7Hz, 2H, COOCH 2 ),4.05(t,J=6.8Hz,2H,COOCH 2 ),2.96(s,2H,CH 2 COOH), 2.83(s, 2H, NHCOCH 2 ), 2.75(t, J=6.6Hz, 4H, CHCH 2 CH),2.39(dd,J=16.3,8.4Hz,2H,CHCH 2 CH 2 ,2H,OCOCH 2 ),2.05(m,8H,CHCHCH 2 ),1.62(...
Embodiment 3
[0090] The compound LA1 is prepared, and the chemical structural formula is as follows:
[0091]
[0092] will LA 2 -COOH (500 mg, 0.67 mmol) was dissolved in dichloromethane, EDCI (206 mg, 1.08 mmol) and HOBt (145 mg, 1.08 mmol) were sequentially added at 0°C and stirred for 5 min, then the mixed solution was moved to room temperature for 3 h to obtain Reaction solution A; 1-(3-aminopropyl)pyrrolidine (85 μL, 0.67 mmol) was dissolved in dichloromethane, triethylamine (280 μL, 2.02 mmol) was added at room temperature, and the reaction was stirred for 1 h to obtain reaction solution B . The reaction solution B was slowly added dropwise to the reaction solution A, and the mixture was stirred at room temperature overnight. After the reaction, the reaction solution was washed twice with an appropriate amount of water, twice with a 10% aqueous citric acid solution, once with saturated brine, dried over anhydrous sodium sulfate, and concentrated by suction filtration to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com