Preparation method of mirabegron and intermediate thereof
A mirabegron, inert gas technology, applied in the field of drug synthesis, can solve the problems of production efficiency restriction, unfavorable production cost, time cost, large amount of auxiliary materials, etc., to ensure product quality and safety, enhance market competitiveness, economical obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
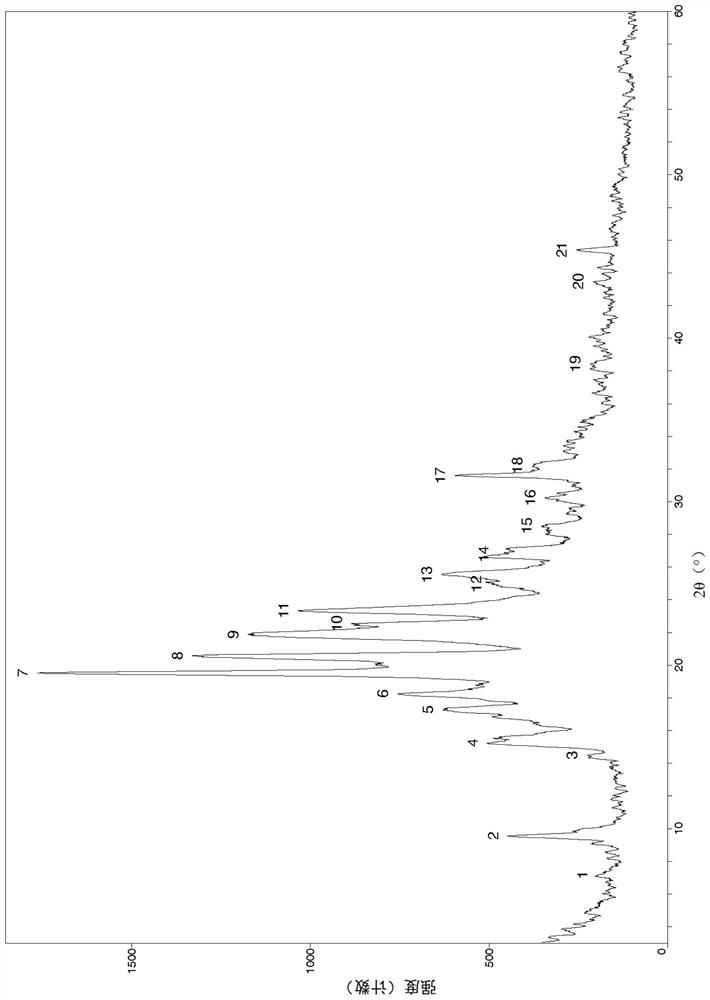
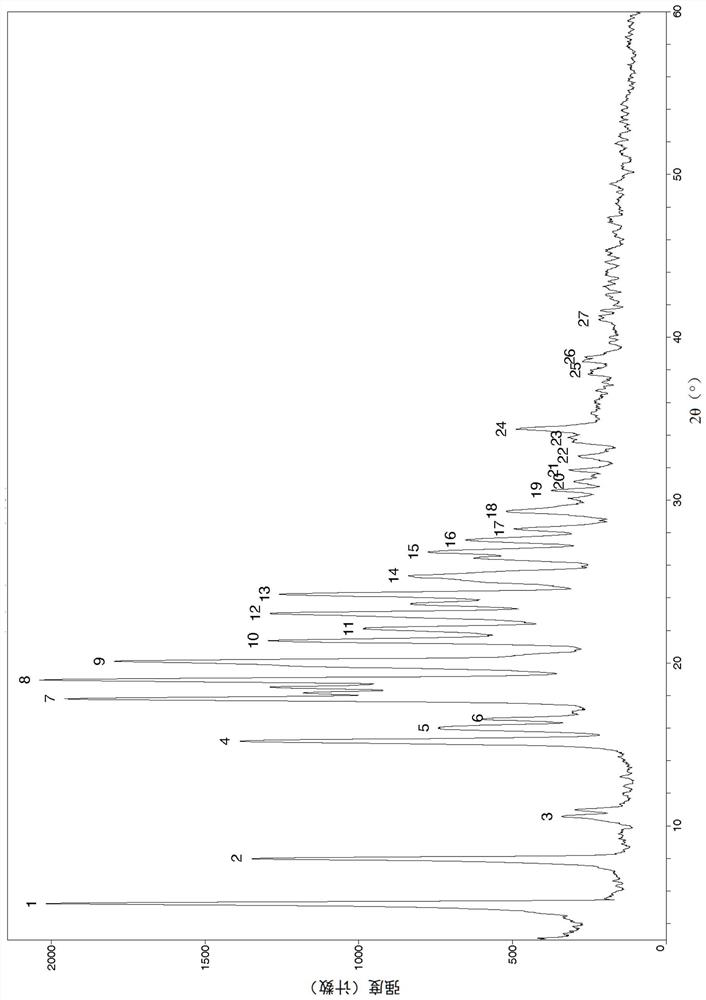
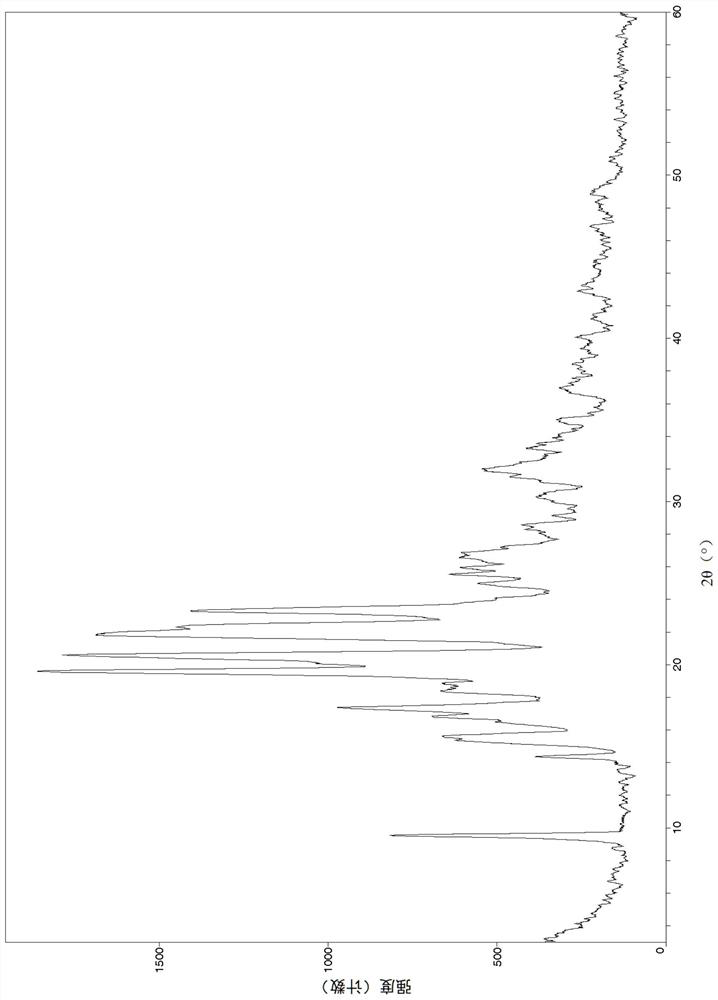
Image
Examples
Embodiment 1
[0113] 1. Preparation of compound 3
[0114]
[0115] Include the following steps:
[0116] ①, in 240kg N,N-dimethylformamide, add 26.02kg (about 171mol) D-mandelic acid (compound 1), 31.5kg (about 155.45mol) 4-nitrophenethylamine hydrochloride (compound 2), 23.12kg (about 171mol) 1-hydroxybenzotriazole, and 35.28kg (about 171mol) N, N-dicyclohexylcarbodiimide, 18.84kg (about 186mol) triethylamine, the temperature of reaction is at 20~25 ℃, stirring and reacting for 5h, after high performance liquid chromatography (HPLC) detects that the reaction is basically complete (the remaining amount of compound 2 is less than or equal to 2%), a reaction solution is obtained;
[0117] 2., in above-mentioned reaction solution, add 360kg ethyl acetate and 604.8kg purified water, stir, pad diatomaceous earth filter afterwards, stand, separatory, get organic phase, the water phase of lower floor is with ethyl acetate (285kg, 171kg) Extract twice, combine the organic phases, add 32.82kg ...
Embodiment 2~4
[0151] In step i of the preparation method of compound 4, the amount of 1,3-dimethylimidazolidinone (DMI) was changed to 0mol, 200mol, and 600mol respectively, that is: in terms of compound 3 (mol), 1,3-dimethylimidazolidinone (DMI) The consumption of diimidazolidinone (DMI) is respectively 0 equivalent, 2.5 equivalent, 7.5 equivalent, other process conditions remain unchanged (identical to embodiment 1), prepare compound 4, its yield and HPLC purity, impurity content are shown in Table 3 .
[0152]
[0153] Table 3, the effect of 1,3-dimethylimidazolidinone (DMI) dosage on the preparation of compound 4
[0154] Example 2 Example 3 Example 1 Example 4 DMI dosage / equivalent 0 2.5 5 7.5 yield 83.0% 86.6% 88.7% 89.2% HPLC purity 96.7% 99.61% 99.6% 99.44% Impurities (337008) 3.1% ND ND ND
[0155] ND means not detected, which means its content is below the detection limit.
Embodiment 5~6
[0157] In step i of the preparation method of compound 4, the dosage of borane dimethyl sulfide was changed to 168 mol and 200 mol, respectively, that is, in terms of compound 3 (mol), the dosage of borane dimethyl sulfide was respectively 2.1 equivalent and 2.5 equivalent. , other process conditions remain unchanged (same as Example 1), compound 4 is prepared, and its yield, HPLC purity and impurity content are shown in Table 4.
[0158] Table 4, the effect of borane dimethyl sulfide dosage on the preparation of compound 4
[0159] Example 5 Example 1 Example 6 Borane dimethyl sulfide amount / equivalent 2.1 2.3 2.5 yield 86.0% 88.7% 88.5% HPLC purity 99.76% 99.6% 99.73% Impurities (337008) ND ND ND
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com