Rare earth precursor, method of preparing same, and method of forming thin film using same
A technology of rare earth elements and ligands, used in the field of vapor deposition compounds, to achieve the effects of excellent thermal stability and volatility, excellent film physical properties, and excellent reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
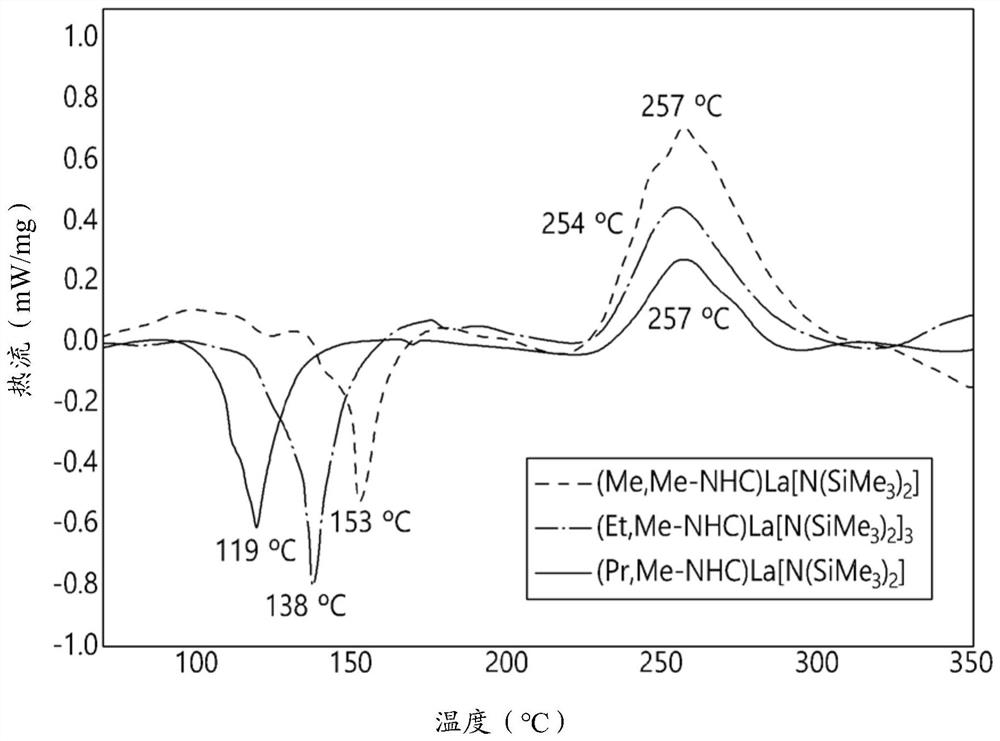
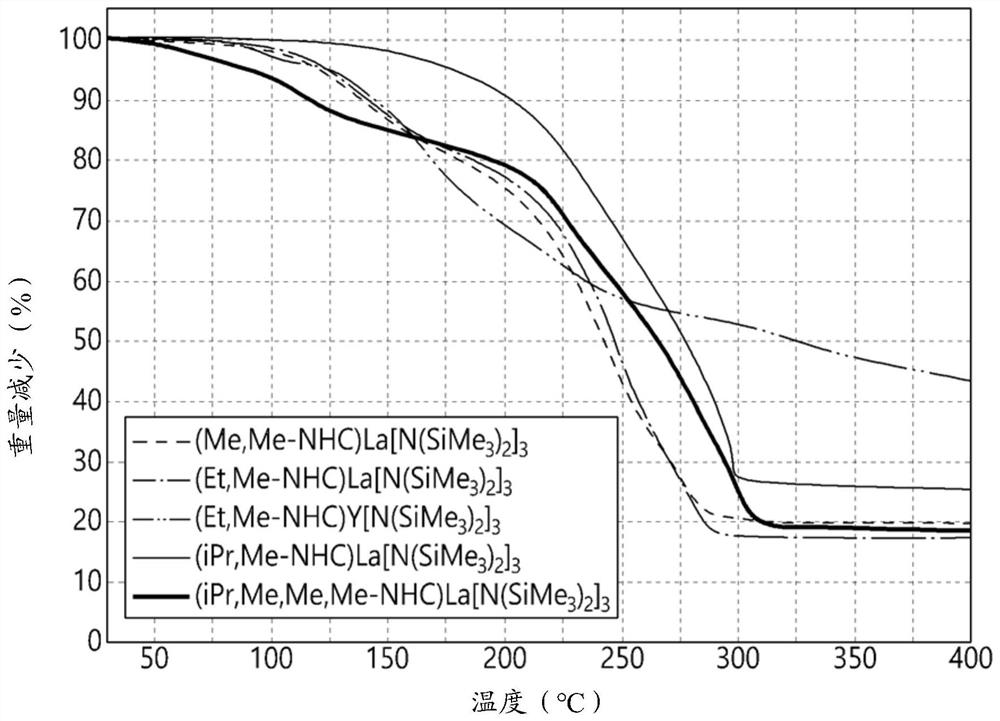
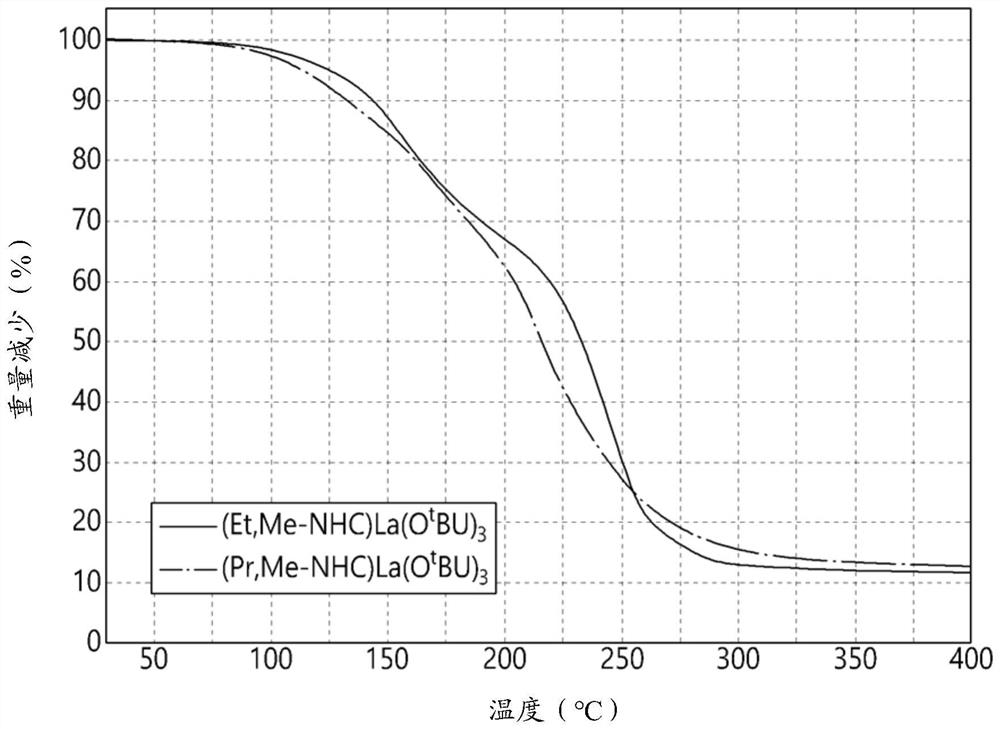
Image
Examples
Synthetic example 1
[0085] (NHC)La[N(SiMe 3 ) 2 ] 3 Synthesis
[0086] Weigh 7.36 g (0.03 mol) of lanthanum(III) chloride and 0.03 mol of alkylimidazolium chloride or bromine (1-R1-3-R2-4-R3-5-R4-imidazolium chloride or bromine) in a flask, and 200 mL of tetrahydrofuran (THF) was added to dissolve the compound. At 0°C, 22 g (0.12 mol) of sodium bis(trimethylsilyl)amide in THF was slowly added to the solution. After that, the reaction was completed by stirring at room temperature for about 16 hours. Then, the solvent and volatile by-products were removed under vacuum. The residue was diluted by adding hexane, the diluted residue was filtered through a filter containing celite, and the filtrate was again dried under vacuum. The solid was redissolved in hexane and recrystallized at -40°C to give a colorless or white crystalline solid.
[0087] The reaction of Synthesis Example 1 is represented by Chemical Reaction Formula 1 below.
[0088] [chemical reaction formula 1]
[0089]
[0090]...
Synthetic example 2
[0091] (NHC)La(O t Bu) 3 Synthesis
[0092] The (NHC)La[N(SiMe) synthesized in Synthesis Example 1 3 ) 2 ] 3 (0.0045 mol) was dissolved in 50 mL of toluene and 1.28 mL (0.0135 mol) of tert-butanol diluted in toluene was added slowly at 0°C. After that, the reaction was completed by stirring at room temperature for about 16 hours. Then, the solvent and volatile by-products were removed under vacuum. The residue was diluted by adding hexane, the diluted residue was filtered through a filter containing celite, and the filtrate was again dried under vacuum. The solid was redissolved in hexane and recrystallized at -40°C to give an orange solid.
[0093] The reaction of Synthesis Example 2 is represented by the following chemical reaction formula 2.
[0094] [chemical reaction formula 2]
[0095]
[0096] In chemical reaction 2, t Bu is tert-butyl and HMDS is hexamethyldisilazane.
Embodiment 1
[0097] (Me,Me-NHC)La[N(SiMe 3 ) 2 ] 3 Synthesis
[0098] According to Synthesis Example 1, (Me,Me-NHC)La[N(SiMe 3 ) 2 ] 3 . In chemical reaction 1, R 1 and R 2 each is methyl, R 3 and R 4 Each is hydrogen, Ln is lanthanum (la), and the synthesized compound is as follows
[0099]Formula 3-1 represents.
[0100]
[0101] The synthesized compound was a colorless crystalline solid with a yield of 70.51%, and the measured 1 The H-NMR peaks are as follows.
[0102] 1 H-NMR (400MHz, C 6 D 6 ): δ0.35(s, 54H), δ3.36(s, 6H), δ5.94(s, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com