Synthesis method of stably dispersed near-infrared Ag2X nanocrystal colloidal solution
A technology of colloidal solution and synthesis method, which is applied in chemical instruments and methods, electric solid devices, semiconductor devices, etc., can solve problems such as the inability to meet the needs of solar cells, and achieve the effect of improving ligand density and increasing binding energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
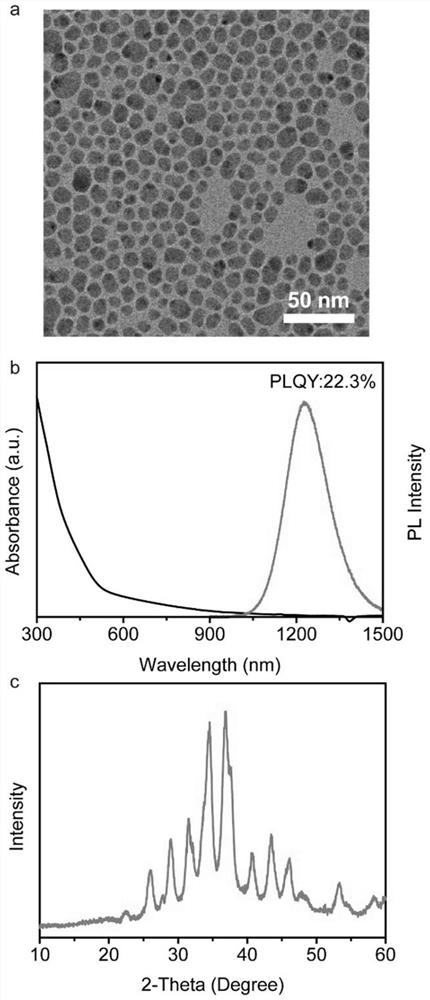
[0033] Example 1: Adding Bi 3+ Modified Ag 2 S nanocrystal synthesis
[0034] In a 50 mL three-neck round bottom flask, 1.0 mmol silver nitrate, 1.0 mmol bismuth chloride, 1.0 mL oleic acid, 1.0 mL oleylamine and 8.0 mL octadecene were added. The flask was heated to 115°C and evacuated for 30 minutes to remove air and moisture from the system. Then, argon was introduced, the temperature was raised to 170° C., 0.45 mmol of p-diphenylthiourea (0.1 M dissolved in diphenyl ether, 4.5 mL) was injected, and the temperature was lowered to room temperature after 30 minutes of reaction. The resulting solution was centrifuged to remove the precipitate, washed with toluene and acetonitrile at least three times, and dispersed in a non-polar solvent.
[0035] like Figure 9 shown in the reference bottle is Ag with no heterovalent cation modification on the surface 2 State of S nanocrystals in a nonpolar solvent, BiCl 3 Add Bi to the bottle 3+ Modified Ag 2 S nanocrystals can be wel...
Embodiment 2
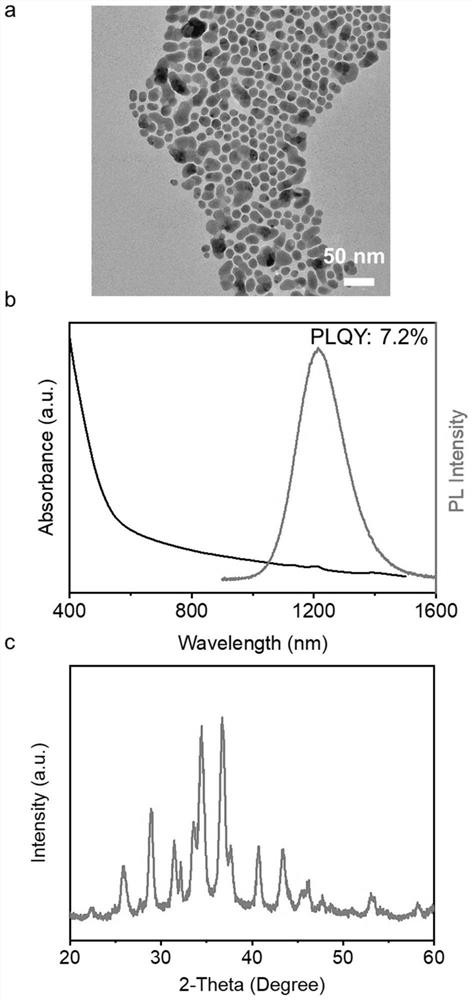
[0036] Example 2: Addition of Zn 2+ Modified Ag 2 S nanocrystal synthesis
[0037] In a 50 mL three-neck round bottom flask, 1.0 mmol silver nitrate, 0.1 mmol zinc chloride, 1.0 mL oleic acid, 1.0 mL oleylamine and 8.0 mL octadecene were added. The flask was heated to 115°C and evacuated for 30 minutes to remove air and moisture from the system. Then, argon was introduced, the temperature was raised to 170° C., 0.45 mmol of p-diphenylthiourea (0.1 M dissolved in diphenyl ether, 4.5 mL) was injected, and the temperature was lowered to room temperature after 30 minutes of reaction. After washing with toluene and acetonitrile at least three times, it is dispersed in a non-polar solvent.
[0038] like Figure 9 shown in the reference bottle is Ag with no heterovalent cation modification on the surface 2 State of S nanocrystals in a non-polar solvent, ZnCl 2 Add Zn to the bottle 2+ Modified Ag 2 S nanocrystals can be well dispersed in non-polar solvents, so this sample can ...
Embodiment 3
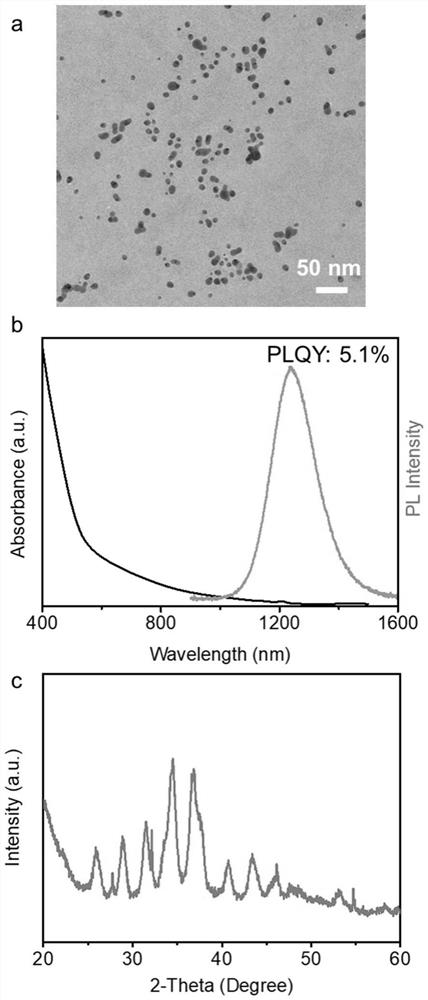
[0039] Example 3: Adding Ga 3+ Modified Ag 2 S nanocrystal synthesis
[0040] In a 50 mL three-neck round bottom flask, 1.0 mmol of silver nitrate, 1 mmol of gallium chloride, 1.0 mL of oleic acid, 1.0 mL of oleylamine and 8.0 mL of octadecene were added. The flask was heated to 115°C and evacuated for 30 minutes to remove air and moisture from the system. Then, argon was introduced, the temperature was raised to 170° C., 0.45 mmol of p-diphenylthiourea (0.1 M dissolved in diphenyl ether, 4.5 mL) was injected, and the temperature was lowered to room temperature after 30 minutes of reaction. After washing with toluene and acetonitrile at least three times, it is dispersed in a non-polar solvent.
[0041] like Figure 9 shown in the reference bottle is Ag with no heterovalent cation modification on the surface 2 State of S nanocrystals in a nonpolar solvent, GaCl 3 Add Ga to the bottle 3+ Modified Ag 2 S nanocrystals can be well dispersed in non-polar solvents, and this ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com