Cis-aconitic anhydride bond adriamycin/human serum albumin (CAD/HSA) composition and preparation method and application thereof
A technology of aconitic anhydride-bonded doxorubicin and human serum albumin, which is applied in the field of pharmaceutical preparations and preparations of doxorubicin-coupled albumin, which can solve cell and tissue damage, toxic side effects, chemotherapy drugs lack of tumor cells or Issues such as tissue selectivity or targeting can achieve the effects of increasing accumulation, reducing heart and kidney toxicity, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
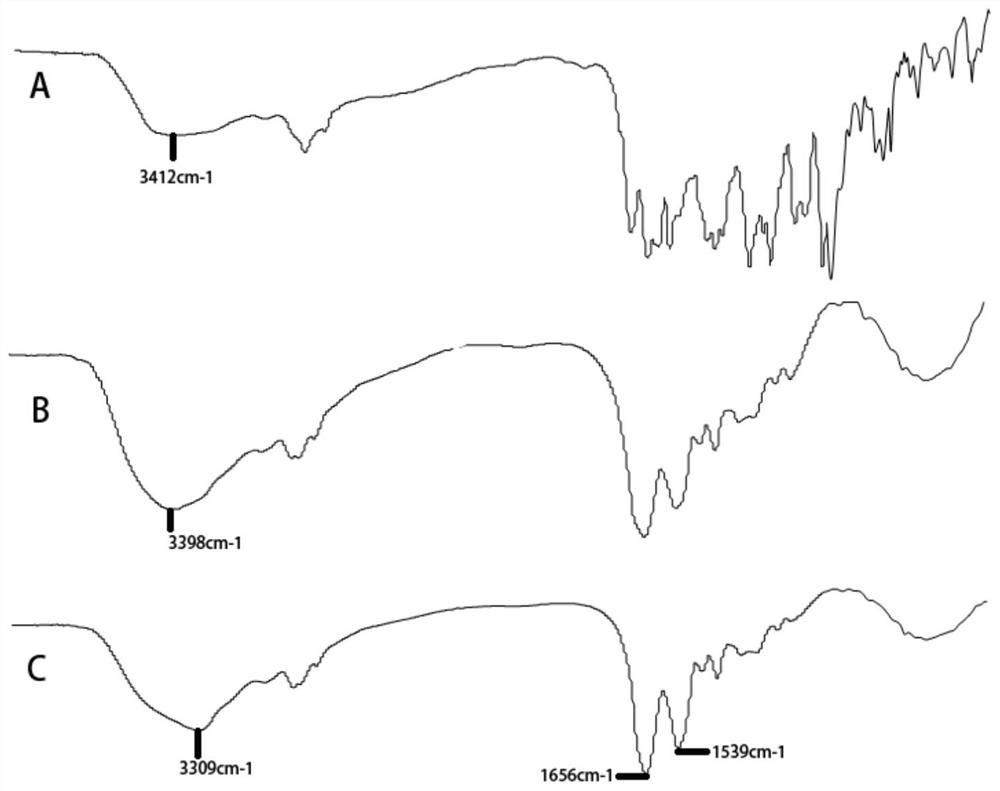
[0027] Example 1 Preparation process and characterization of CAD / HSA
[0028] (1) Preparation of cis-aconitic anhydride-bonded doxorubicin / human serum albumin (CAD / HSA) composition, the molar ratio of the cis-aconitic anhydride-bonded doxorubicin / human serum albumin (CAD / HSA) The ratio is 20:10:1.
[0029] Take 7mg cis-aconitic anhydride doxorubicin (CAD), 8mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide salt (EDCI), 6mg N-hydroxysuccinimide (NHS) Dissolve in 3mL pH7.4 phosphate buffer and stir for 4 hours in the dark; then dissolve 66.7mg of human serum albumin (HSA) in 2mL pH7.4 phosphate buffer, add human serum to the reaction solution albumin (HSA) solution, stirred at room temperature for 24 hours, and finally centrifuged at 4000 rpm for 5 min to take the supernatant, dialyzed with a 25kD dialysis bag for 24 hours, and separated free cis-aconitic anhydride-bonded doxorubicin (CAD) with a SepHadex 25 column. The purified product was freeze-dried to obtain cis-aconitic an...
Embodiment 2
[0034] Example 2 Determination of DOX coupling ratio in CAD / HSA
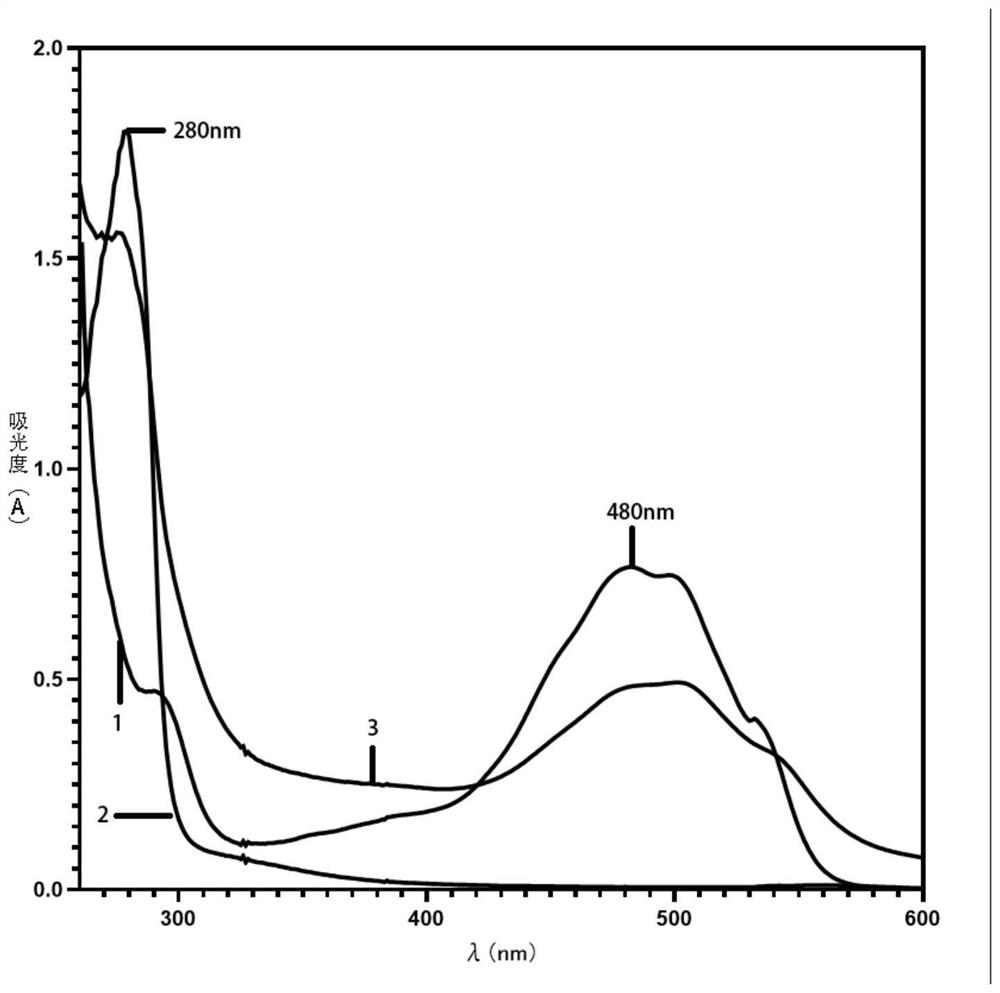
[0035] Determination of DOX content by trypsin hydrolysis method: Weigh 5 mg CAD / HSA, add 1 mL of 250 μg / mL bovine trypsin, put it in a water bath at 37 °C for 5 hours, centrifuge at 10,000 rpm / min, and take the supernatant after 2 minutes. , and then use an ultraviolet spectrophotometer to measure the absorbance value at 490nm, and substitute it into the 490nm DOX ultraviolet standard curve to calculate the content.
[0036] Table 1 Content of DOX in 5 batches of CAD / HSA
[0037]
[0038] In order to determine the coupling ratio of DOX, a regression curve was used to determine the DOX content in the samples after hydrolysis. The regression curve of DOX Y=0.01835X(μg / mL)+0.01558(R 2 =0.9992).
[0039] Five batches of CAD / HSA samples were measured, and the calculated molar ratio was DOX:HSA=2-3:1 (n=5). It shows that HSA and DOX can be coupled within a certain range.
Embodiment 3
[0040] Example 3 In vitro acid-sensitive drug release experiment of CAD / HSA
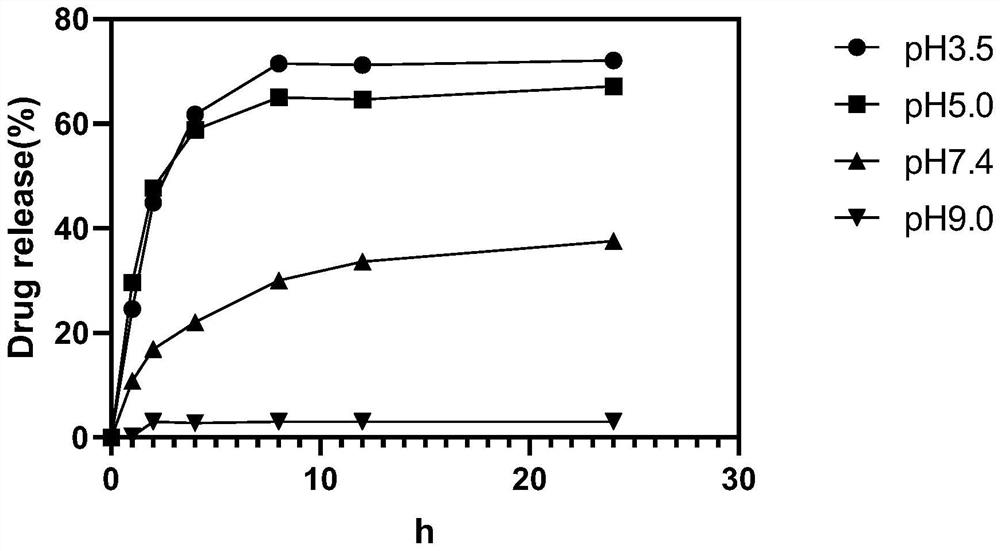
[0041]Weigh 5mg CAD / HSA respectively, dissolve with 1mL buffer solution of pH 3.5, 5.0, 7.4, 9.0 in turn and put it into a 25KDa dialysis bag, add the dialysis bag in turn to a beaker containing a 50mL system of corresponding pH, and place it in the dialysis bag. In a constant temperature shaker at 37°C and 70rpm / min, take 1mL of the external buffer of each dialysis bag at 1, 2, 4, 8, 12, and 24 hours respectively, take three times in parallel, and then make up the corresponding pH buffer to 50mL, The F value of the samples at each time point was measured at 493 nm with a spectrofluorometer, and substituted into the DOX spectrofluorometer standard curve (Y=138.6X+21.45, R 2 =0.9961), calculate the content of DOX at each time point, and draw the in vitro release curve of CAD / HSA to study the sensitive response of CAD / HSA to different pH.
[0042] from image 3 It can be seen that CAD / HSA releases mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com