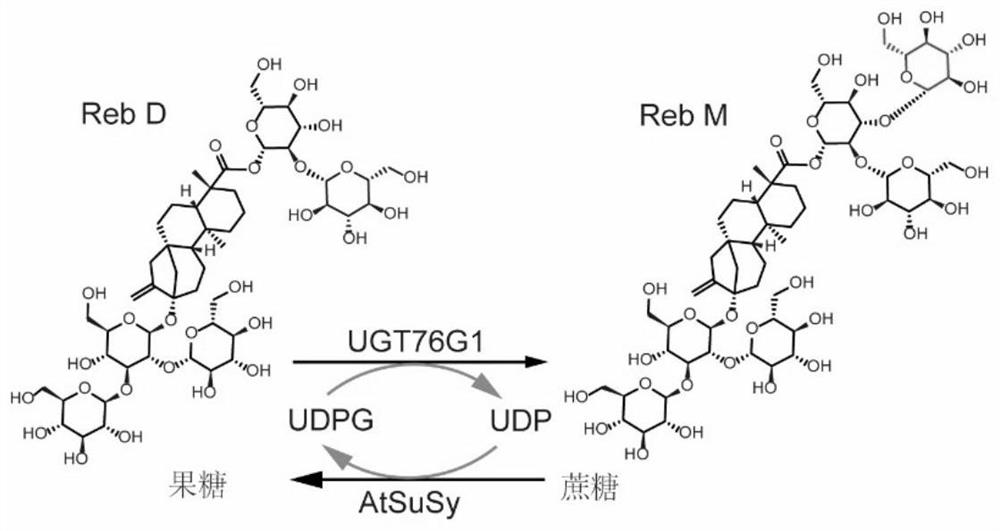
Method for efficiently biosynthesizing rebaudioside M by using glycosyltransferase UGT76G1 mutant
A technology of glycosyltransferase and mutants, which is applied in the field of enzyme engineering to achieve the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Acquisition of glycosyltransferase UGT76G1 gene and construction of mutants
[0041] The amino acid sequence (accession number: Q6VAB4) and nucleic acid sequence (accession number: PRO_0000434465) of the stevia-derived glycosyltransferase UGT76G1 were downloaded from Genbank, and the mutation point T284S was added. Gene synthesis and codons were performed by Yixin Biotechnology Co., Ltd. After optimization and ligation to the polyclonal restriction site of the vector pETDuet-1, the recombinant plasmid pETDuet-1-UGT76G1-T284S was obtained. The sucrose synthase AtSuSy amino acid sequence (Accession No.: NP_001031915) and its nucleic acid sequence (Accession No.: NM_001036838.2) derived from Arabidopsis thaliana were downloaded from Genbank, and E. coli-preferred codon optimization was performed by Yixin Biotechnology Co., Ltd. and gene synthesis.
[0042] Take recombinant plasmid pETDuet-1-UGT76G1-T284S as template, use primers M88L-F / M88L-R and L200A-F / L200A-R...
Embodiment 2
[0046] Example 2 Induction and expression of recombinant strains and purification of target protein
[0047] The recombinant strains E.coli BL21(DE3)pETDuet-1-UGT76G1-T284S and E.coli BL21(DE3)pETDuet-1-UGT76G1-T284S / M88L / L200A constructed in Example 1 were inoculated into 100 μg / mL ampicillin, respectively. 1L TB liquid medium for penicillin (12g / L peptone, 24g / L yeast powder, 5g / L glycerol, 2.32g / L KH 2 PO 4 , 12.53g / L K 2 HPO 4 ) and cultivated to OD at 115 rpm and 37°C 600 After the temperature was 0.6-0.8, the culture temperature was lowered to 18°C, and isopropyl-β-thiogalactoside (IPTG) with a final concentration of 0.1 mM was added to induce culture for 8 hours.
[0048] Centrifuge the induced expression bacterial liquid (7000 rpm, 7 min, 4 °C), discard the supernatant, and collect the bacterial cells. The cells were resuspended in lysis buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl, 10 mM imidazole, 10% glycerol) at 1 g of cells per 10 mL of lysis buffer. The high-...
Embodiment 3
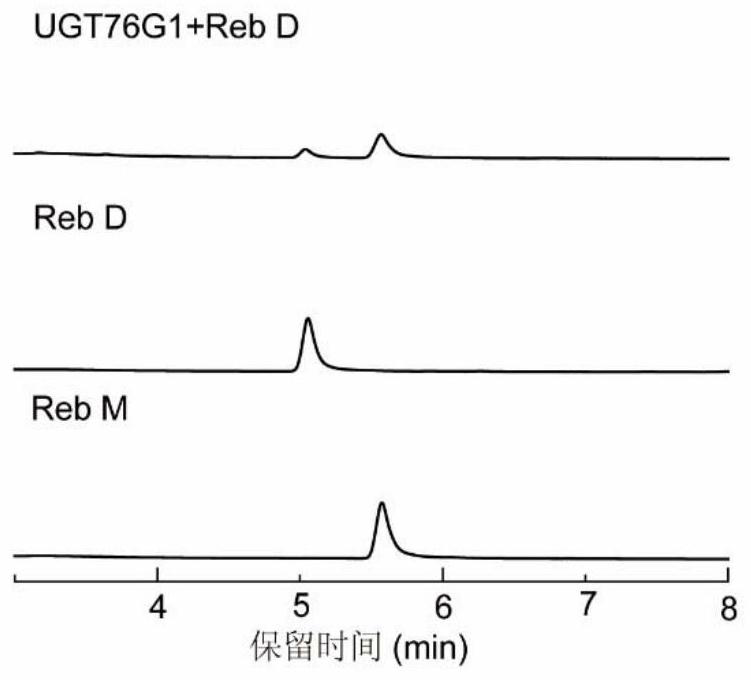
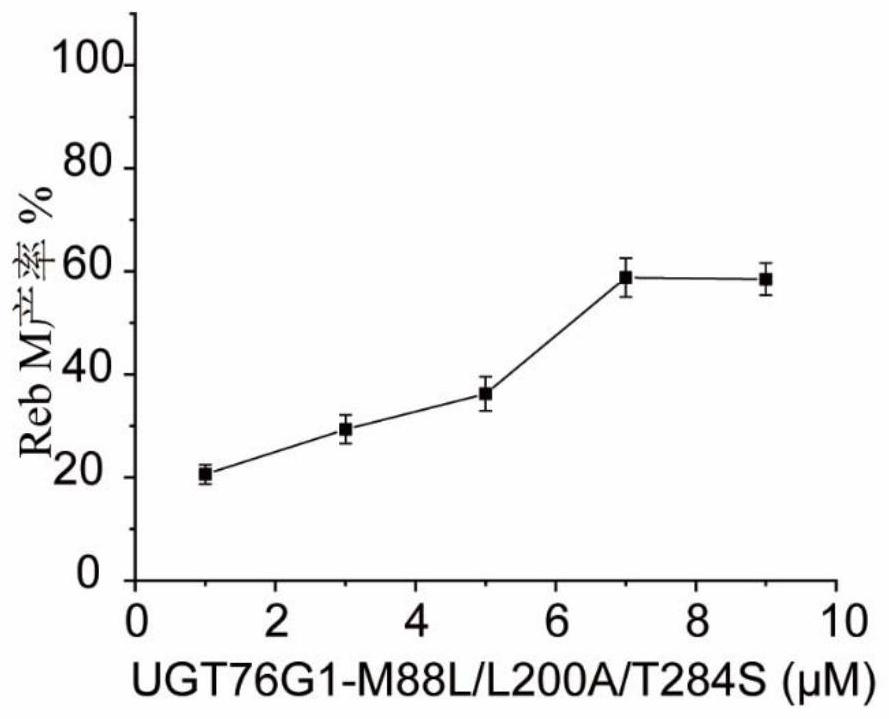
[0051] Example 3 UGT76G1-T284S and UGT76G1-T284S / M88L / L200A catalyze the glycosylation of Reb D to synthesize Reb M
[0052] The purified wild-type enzyme obtained in Example 2 and the mutant UGT76G1-T284S / M88L / L200A were subjected to glycosylation reaction.
[0053] The glycosylation reaction was carried out in a 200 μL reaction system, the reaction system was as follows: 50 mM Tris-HCl pH 8.0, 5 mM UDPG, 2 mM Reb D, the concentration of the pure enzyme UGT76G1-T284S or UGT76G1-T284S / M88L / L200A obtained in Example 2 was 2.5 μM. The reaction was carried out at 35°C for 10 min. After the reaction, heated at 95 °C for 10 min, diluted with 3 times the volume of methanol, centrifuged at 20,000 × g for 5 min, filtered with a 0.22 μM filter membrane, and then carried out ultra-high performance liquid chromatography (UPLC) for detection and analysis ( figure 2 ). UPLC adopts the BEH C18 1.7μM reverse column of waters company, the injection volume is 4μL, the column temperature is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com