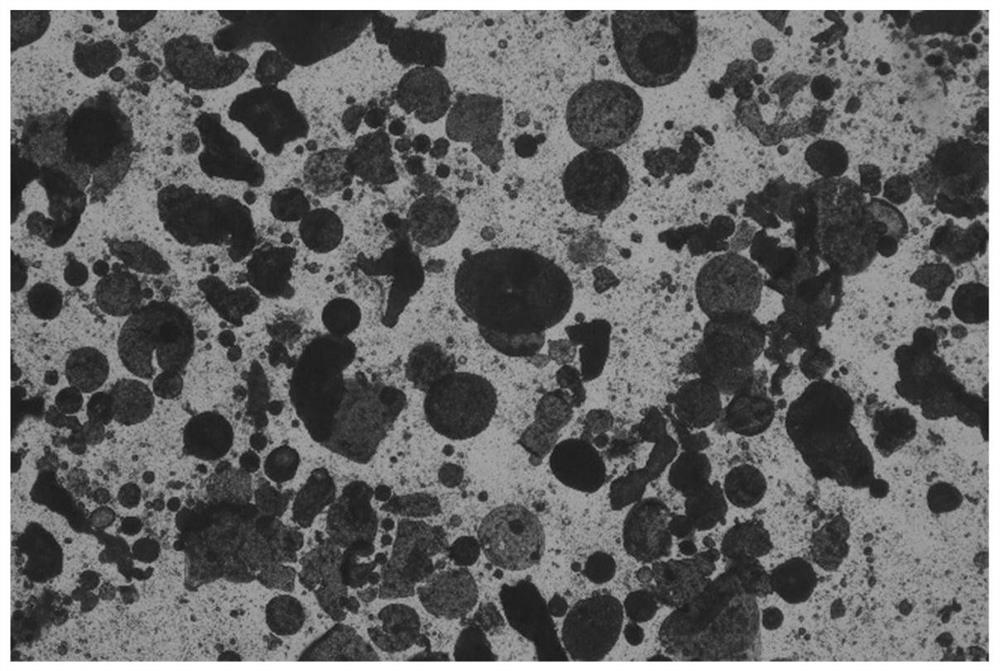
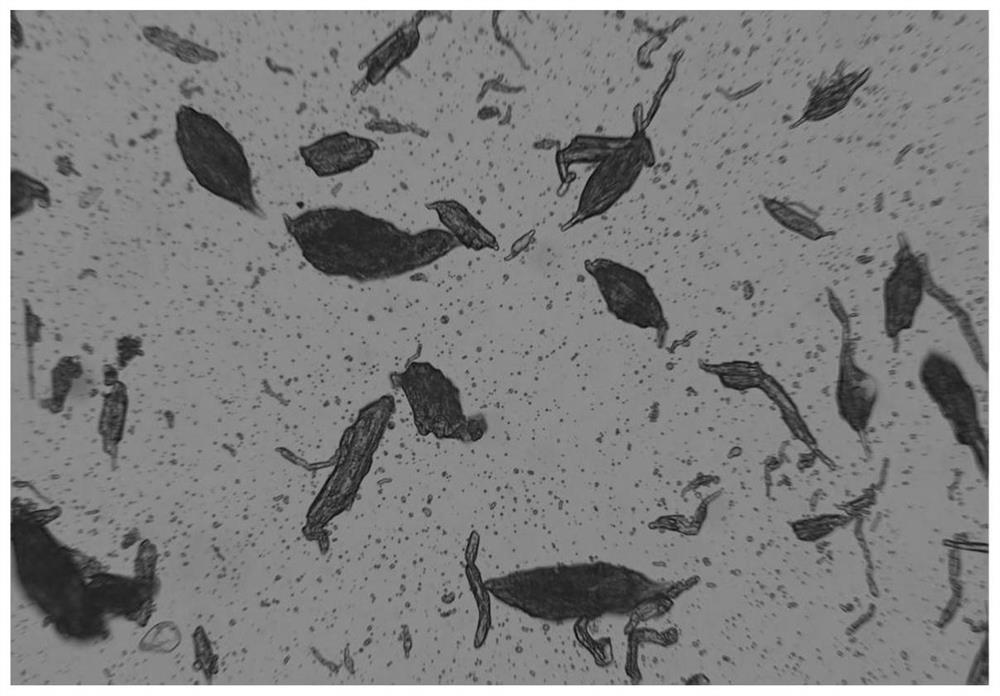
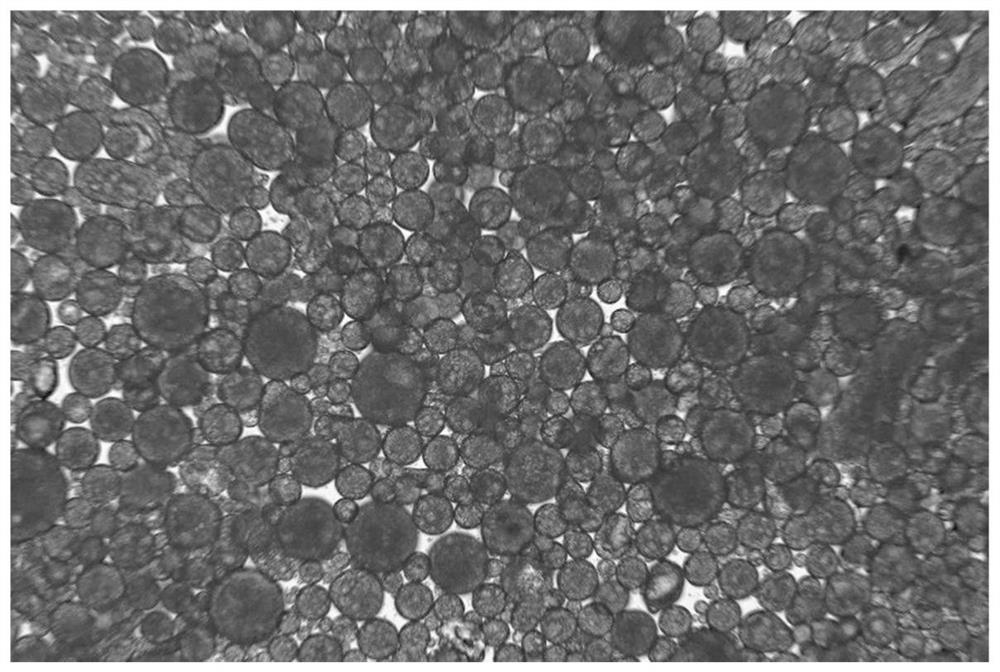
Preparation method of PLGA (poly (lactic-co-glycolic acid)) drug sustained release microspheres
A technology for slow-release microspheres and drugs, which is applied in the production of pharmaceutical formulations, inactive medical preparations, and bulk chemicals. It can solve the problems of low PLGA concentration, long curing time, etc. The effect of easy control and shortening of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of PLGA drug sustained-release microspheres by conventional emulsifying solvent evaporation method
[0050] The microspheres were prepared according to the recipe in Table 1.
[0051] 1. Preparing the solidified phase: Weigh a certain amount of surfactant in a beaker containing ultrapure water and wait for it to be dissolved for later use.
[0052] 2. Preparation of oil phase: Add a certain concentration of PLGA and dexamethasone small molecule drug into a beaker containing an organic solvent, and turn on the stirring device to stir the oil phase to obtain a uniform drug water-in-oil emulsion or drug particle suspension system .
[0053] 3. Under mechanical stirring, add the oil phase to the water phase to emulsify and solidify.
[0054] 4. After the solidification is completed, solid-liquid separation, centrifugation and washing are carried out by means of centrifugation to obtain concentrated microspheres.
[0055] 5. Transfer the washed conce...
Embodiment 2
[0070] Example 2 Preparation of PLGA drug sustained-release microspheres by polymer-assisted phase inversion method
[0071] Referring to Table 2, select different types of interface inversion regulators, and prepare PLGA drug sustained-release microspheres by polymer-assisted phase inversion method. The preparation process is as follows:
[0072] 1. Preparation of water phase: Weigh a certain amount of interface inversion regulator A or / and B, respectively, and dissolve them in a beaker containing ultrapure water for use.
[0073] 2. Preparation of solidified phase: prepare 10% poloxamer aqueous solution (W / V), and set aside.
[0074] 3. Oil phase: Dissolve 3g PLGA and 1.5g dexamethasone in a beaker containing 10ml of ethyl acetate solvent, turn on the stirring device, and continue stirring to obtain a uniform drug water-in-oil emulsion or drug particle suspension system.
[0075] 4. Through the peristaltic pump, the water phase is added to the oil phase at a fixed flow rate...
Embodiment 3
[0089] On the basis of Example 2, when poloxamer and mannitol with a mass ratio of 1:10 were used as interface modifiers, the concentration of PLGA in the oil phase was changed, and the preparation of PLGA drug sustained-release microspheres was carried out. The preparation steps :
[0090] 1. Preparation of water phase: Weigh 5g of poloxamer and 50g of mannitol respectively and dissolve them in a beaker of 250ml of ultrapure water, set aside.
[0091] 2. Preparation of solidified phase: prepare a 10% poloxamer aqueous solution for use.
[0092] 3. Oil phase: Dissolve a certain amount of PLGA and triamcinolone acetonide small-molecule drug in a beaker containing an organic solvent, turn on the stirring device, and continuously stir to obtain a uniform drug particle suspension system.
[0093] 4. Through the peristaltic pump, the water phase is added to the oil phase at a certain flow rate, and the semi-solidified microspheres are rapidly formed.
[0094] 5. Then add the soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com