Pichia pastoris strain for expressing osteopontin
A Pichia pastoris, osteopontin technology, applied in fungi, microorganism-based methods, polypeptides containing localization/targeting motifs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Construction of embodiment 1 expression plasmid
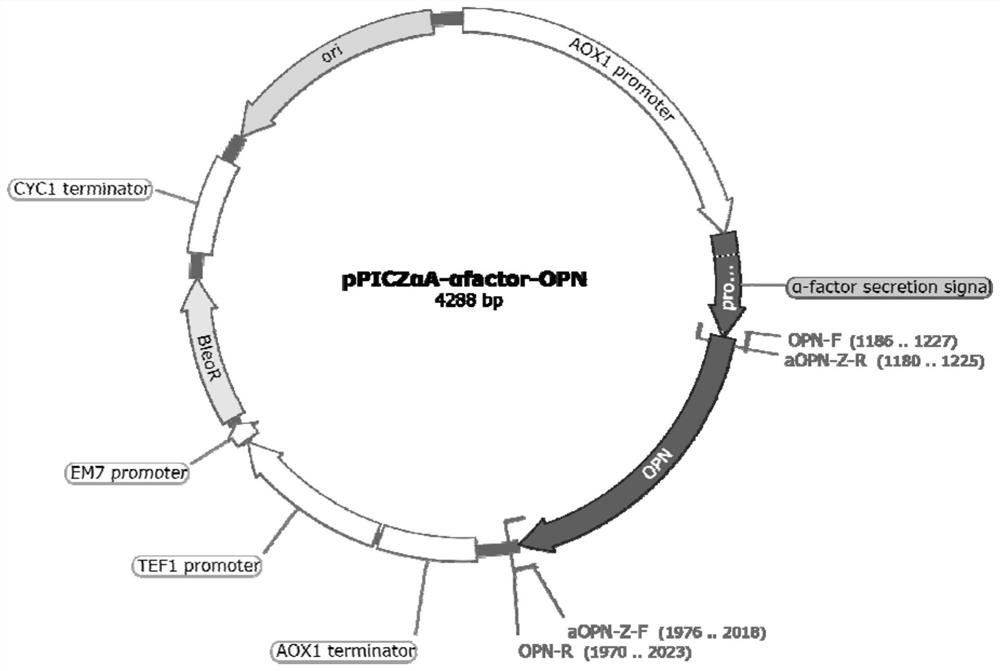
[0034] (1) Using the artificially synthesized OPN gene as a template, use the primers OPN-F and OPN-R in Table 1 to amplify osteopontin, use the plasmid pPICZαA stored in the laboratory as a template, and use the primers aOPN- The α-signal peptide was amplified from Z-F and aOPN-Z-R. The amplified osteopontin and α-signal peptide were connected to the pPICZαA plasmid digested with EcoRI and NotI to obtain the recombinant plasmid pPICZαA-αfactor-OPN. Plasmid map such as figure 1 shown.
[0035] The PCR system is:
[0036] Table 1 PCR reaction system
[0037]
[0038]
[0039] The PCR conditions are:
[0040] Table 2 PCR amplification conditions
[0041]
[0042] Design the pPICZαA-αfactor-OPN recombinant plasmid, the plasmid map and primer sequences are as follows figure 1 and shown in Table 3.
[0043] Table 3 pPICZαA-αfactor-OPN primer sequence
[0044]
Embodiment 2
[0045] The construction of embodiment 2 recombinant Pichia pastoris
[0046]The recombinant plasmid pPICZαA-αfactor-OPN in Example 1 was linearized with Dra I, and 80 μL of Pichia pastoris X33 competent cells were mixed with 10 μL of the linearized recombinant plasmid, and transferred to a 0.2 cm electrotransformation cup, and ice-bath the electrotransformation cup containing the mixed solution for 5 min. Adjust the parameters of the electrotransformer, put it in the Pichia pastoris gear, the voltage is 1.5 kV, the capacitance is 25 microfarads, the resistance is 200 ohms, and the time is about 5 milliseconds. After the electric shock, quickly add 1mL of 1M sorbitol solution pre-cooled on ice to the transformation cup, gently pipette to mix, and transfer the mixture to a centrifuge tube. Incubate at 30°C for 1-2h, and centrifuge at 2000g for 5min. Discard 800 μL of the supernatant, blow the remaining bacteria evenly, spread it on a YPD plate, and culture it in a 30°C incubat...
Embodiment 3
[0047] Example 3 Fermentative production of osteopontin in recombinant Pichia pastoris
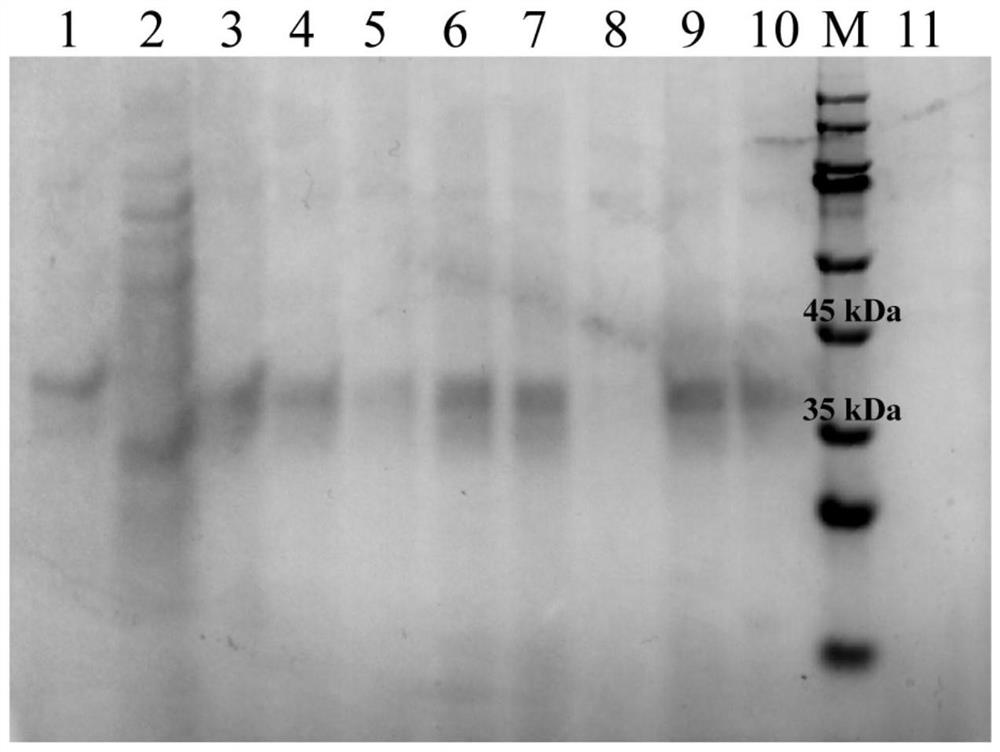
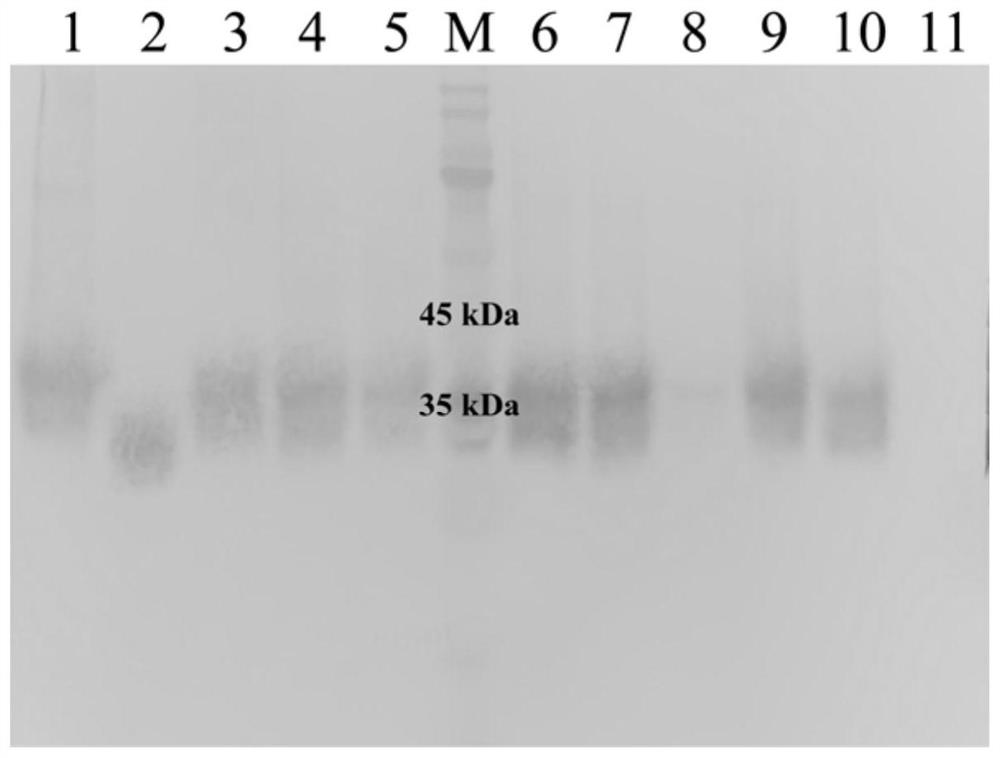
[0048] Pick a single colony from the YPD plate in Example 2 and inoculate it into a round-bottomed test tube containing 2mL of YPD medium for overnight culture. Take 1mL of the overnight culture and inoculate it into 25mL of BMGY medium for shake-flask fermentation at 30°C and 220rpm cultured to OD 600 At about 90, 4°C, 3500g, centrifuge for 10min, collect the cells, discard the supernatant, and wash twice with sterile water, centrifuge under the same conditions after each wash, and take the supernatant. The cells were resuspended in BMMY medium, and the expression was induced at 30°C and 220rpm, and methanol was added every 24h to a final concentration of 1%. After being induced by methanol for 7 days, the supernatant was collected by centrifugation, concentrated by centrifugation, and quantitatively analyzed for osteopontin by SDS-PAGE and Western Blot. The result is as figure 2 , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com