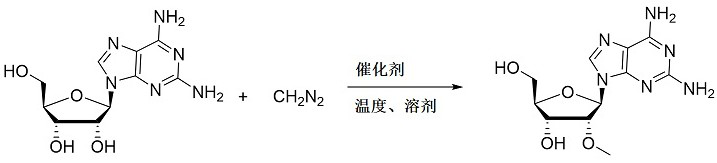
Continuous preparation process of 2 '-O-methyl-2-amino adenosine
An aminoadenosine and preparation process technology, applied in the preparation of sugar derivatives, sugar derivatives, organic chemistry, etc., can solve the problems of low yield and complicated purification, achieve process safety, simplify post-processing purification steps, reduce The effect of chemical reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
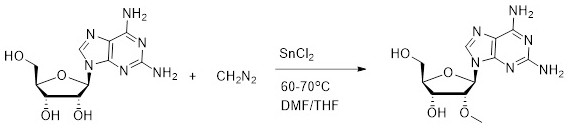
[0019] like figure 2 As shown, the continuous preparation process of 2'-O-methyl-2-aminoadenosine, the steps include:
[0020] (1) Dissolve 282.3 g of 2-aminoadenosine in 1411 ml of N,N-dimethylformamide, add 11.3 g (0.05 equivalent) of stannous chloride dihydrate to prepare a homogeneous solution; 0.2 mol of diazonium Methane was dissolved in 15L tetrahydrofuran solution, wherein diazomethane was prepared as needed and prepared as needed;
[0021] (2) Automatic feeding, 2-aminoadenosine is fed from pump A at a rate of L=5.6g / min, and diazomethane is fed from pump B at a rate of L=24.0g / min, entering the coil reaction The reaction is carried out in the reactor, the temperature of the coil reactor is controlled at 60-70 ° C in the water bath, the retention time is 20 minutes, the reaction product is received at the discharge port, the activated carbon is decolorized, and after concentration, the product 2'-O-methyl-2-amino acid is obtained by crystallization. glycoside, whit...
Embodiment 2
[0023] (1) Equipment specifications: automatic control system, 100ml PFE reactor, 50ml plunger pump, 5000g balance, 1000ml feeding bottle.
[0024] (2) Solution A: 2-aminoadenosine + 5V N,N-dimethylformamide + 0.05equiv. stannous chloride, feed rate L=0.7g / min;
[0025] Solution B: diazomethane tetrahydrofuran solution (0.2mol / L), feed rate L=3g / min, and the molar ratio of 2-aminoadenosine to diazomethane is controlled at about 1:1.25;
[0026] (3) Reaction conditions: use a coiled tube reactor, external bath at 60-70°C, retention volume: 100ml, retention time RT=21min, post-treatment to obtain 9.0g of product with a yield of 91% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com