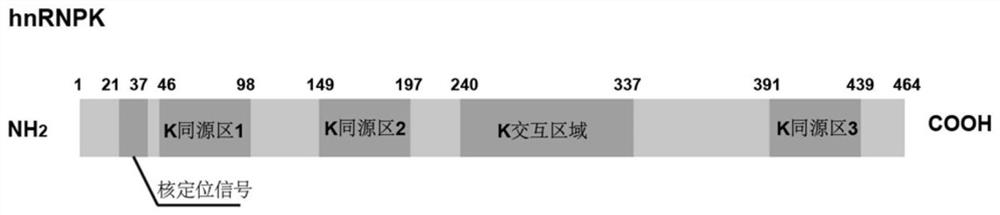
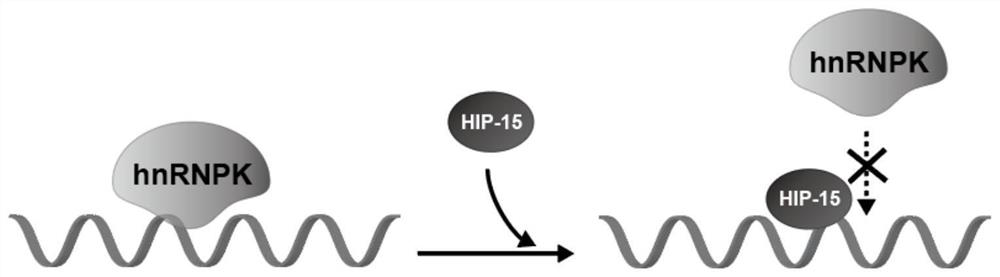
Polypeptide HIP-15 capable of antagonizing hnRNPK protein RNA binding activity and application thereof
A technology that combines activity and protein, applied to polypeptides, hybrid peptides, peptide/protein components containing positioning/targeting motifs, etc., to achieve the effects of inhibiting cell proliferation, inhibiting leukemia, and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of embodiment 1 antitumor polypeptide
[0033] Synthesize an anti-tumor polypeptide by solid-phase synthesis, which includes a tumor cell killing domain and a membrane-penetrating domain, wherein the tumor cell killing domain sequence is TIKLFQECCPHSTDR (SEQ ID No.1), and the membrane-penetrating domain sequence is YGRKKRRQRRR (SEQ ID No.2), the transmembrane domain is connected to the N-terminal of the tumor cell killing domain, the obtained sequence is: the amino acid sequence is YGRKKRRQRRR-TIKLFQECCPHSTDR (SEQ ID No.3), named HIP-15. For the convenience of research, we linked FITC labeled with fluorescein isothiocyanate to the C-terminus of the anti-tumor polypeptide, and linked biotin-labeled Biotin to the N-terminus.
[0034] The tumor suppressor polypeptide HIP-15 synthesized by the biological company has been tested by High Performance Liquid Chromatography (HPLC), and the purity reaches 97.3%. The polypeptide was dissolved in sterile PBS buffer (Sigm...
Embodiment 2
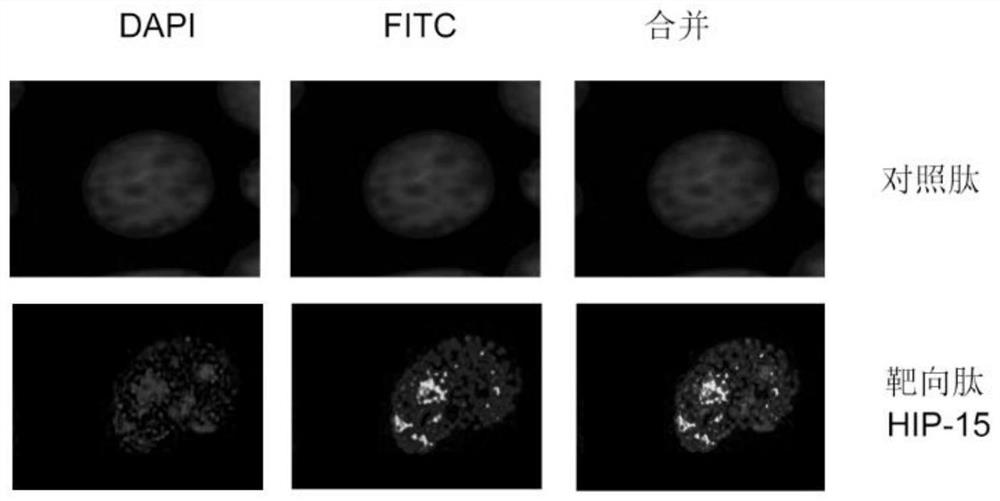
[0037] Example 2 Detection of cellular localization of HIP-15
[0038]Human leukemia cell line HL-60 (China Center for Type Culture Collection) was used for detection. Suspension cells grown in logarithmic phase were added to 24-well plates at approximately 15,000 cells per well. Cultured in 10% fetal bovine serum (HyClone Company), RPMI1640 medium (Gibco Company), and 30 μM polypeptide HIP-15 was added, and maintained for 48 hours. A staggered peptide with the same amino acid composition as the polypeptide was randomly rearranged as a control peptide. Aspirate the cell suspension in each well, adjust the speed of the centrifuge (Fresco21, ThermoFisher Company) to centrifuge at 1000 rpm for 5 minutes, add 500 μl of 4% paraformaldehyde (Sigma-Aidrich Company) after washing with 1x PBS, and fix the cells at room temperature for 20 minutes , discard the paraformaldehyde, wash twice with 1x PBS, 5 min each. Add 300 μl of 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aidrich Compan...
Embodiment 3
[0040] Example 3 MTT colorimetric analysis to detect the inhibitory effect of HIP-15 on the growth of leukemia cells
[0041] The effect of the polypeptide on the proliferation activity of different tumor cells was detected by MTT method. The human leukemia cell line HL-60 was used for detection. A staggered peptide with the same amino acid composition as the polypeptide was randomly rearranged as a control peptide. HL-60 cells in the logarithmic growth phase were taken, and 5000 cells were planted in each well of a 96-well cell culture plate, and 10 replicate wells were set for each sample. After 24 hours, the HIP-15 polypeptide was added. The concentrations of the polypeptide and its control polypeptide were 10 μM, 15 μM, 20 μM, and 30 μM, respectively; the action time was 24 hours, 48 hours, and 72 hours, respectively. After reaching the calibration time, centrifuge in a 96-well plate centrifuge (Eppendorf), discard the culture supernatant, and wash the sample wells wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com