Fully human monoclonal antibody for resisting novel coronavirus and application of fully human monoclonal antibody
A monoclonal antibody, coronavirus technology, applied in the direction of antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve the problems of limited plasma sources of recovered patients, the risk of disease transmission between batches, and the inability to popularize and apply, and achieve inhibition. The effect of virus infection on host cells, good druggability, high neutralization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
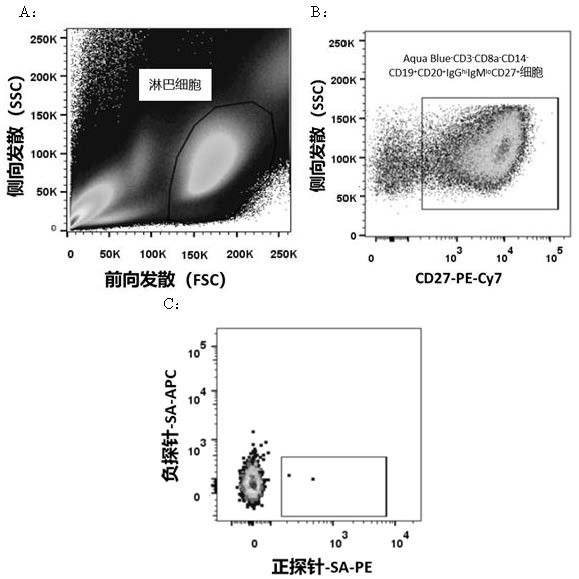
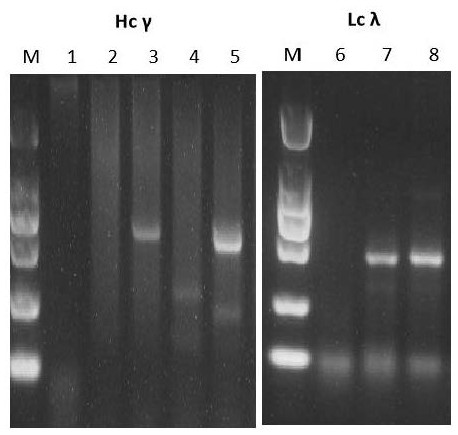
[0064] Example 1: Screening and Preparation of Human Anti-SARS-CoV2 Monoclonal Antibody
[0065] 1.1 Labeling of probes
[0066] The required SARS-CoV2-related specific positive and negative probes are conjugated with fluorescent dyes, and the process is as follows:
[0067] 1.1.1 AviTag fusion protein biotinylation kit (Avidity, BirA500) was used for biotin labeling of probes. The reaction system was prepared according to the steps described in the manual, and incubated at 30 °C for 30 min.
[0068] 1.1.2 Use a 30 kDa ultrafiltration tube to exchange the reaction product with 1× PBS (pH 7.4) to remove excess biotin.
[0069] 1.1.3 Gradually add 1 / 5 molar equivalent of streptavidin-phycoerythrin (SA-PE) or streptavidin-allophycocyanin (SA-APC) to the biotin label at intervals of 20 min until the molar ratio of SA-PE or SA-APC to biotin-labeled probe reached 1:1, and incubated at 4 °C with gentle shaking to prepare positive and negative probes labeled with fluorescein: Ag(-...
Embodiment 2
[0100] Example 2: Analysis of the binding activity of monoclonal antibody YK-CoV2-03 to RBD protein
[0101] Antibody-antigen binding activity analysis using Gator label-free biomolecular analyzer
[0102] 2.1. GNTi cells were used to express RBD, and after purification by nickel column and molecular sieve, RBD was biotinylated according to the steps indicated in the instructions of DSB-X™ Biotin Protein Labeling Kit (Invitrogen™, D20655).
[0103] 2.2 Dilute the biotinylated RBD 600 times, add 3 μL of RBD to 2000 μL of HBS-EP buffer and mix, and load the diluted RBD at 200 μL per well into a 96-well sample plate.
[0104] 2.3 Use HRV 3C protease to cut the monoclonal antibody into Fab fragments, use affinity chromatography and molecular sieve to purify the obtained antibody Fab, after diluting the antibody Fab by 1000 times, continue to perform gradient dilution according to 1:3, a total of 4 dilutions (36.2nM, 12.1 nM, 4.02 nM and 1.34 nM), take 200 μL of each dilution samp...
Embodiment 3
[0109] Example 3: Analysis of the binding ability of monoclonal antibody YK-CoV2-03 to competitively block SARS-CoV2 virus RBD and receptor ACE2
[0110] Analysis of Antibody Blocking Binding Ability of SARS-CoV2 Virus RBD and Receptor ACE2 Using Gator Non-labeled Biomolecular Analyzer
[0111] 3.1 Expi-CHO cells were used to express the receptor ACE2, and after purification by nickel column and molecular sieve, ACE2 was biotinylated according to the steps shown in the instructions of DSB-X™ Biotin Labeling Kit (Invitrogen™, D20655). The biotinylated ACE2 was diluted to 40 μg / mL with a molar concentration of 570 nM.
[0112] 3.2 Dilute RBD to 30 μg / mL, the molar concentration is 1000 nM, dilute the antibody to 0.2 mg / mL, and mix with RBD at a molar ratio of 1:1. The molar amount of antibody in the prepared mixture is greater than that of ACE2 .
[0113] 3.3 During the detection process, each experiment was first equilibrated in the HBS-EP buffer of the probe card for 120 s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com