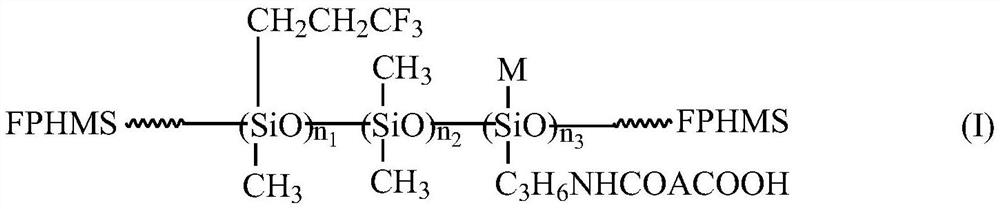
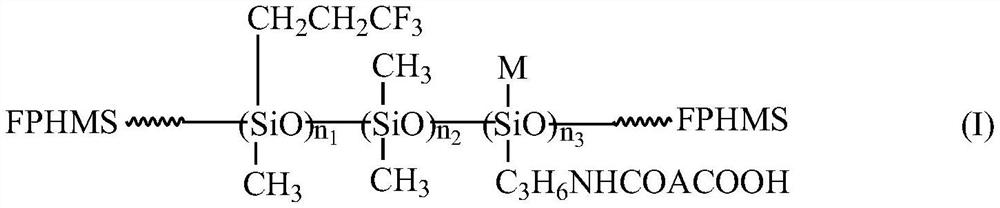
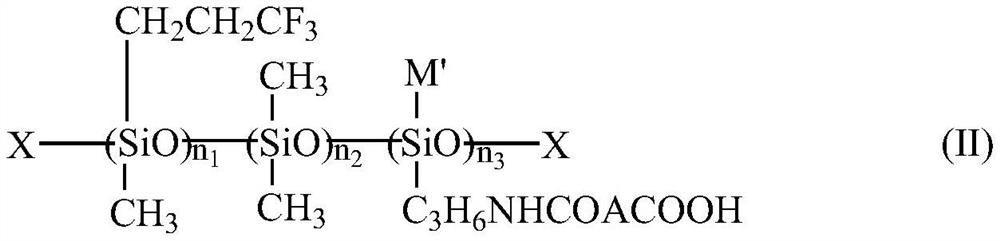Organic fluorosilicone emulsion release agent and preparation method thereof
A technology of release agent and silicone emulsion, applied in the field of functional additives, can solve the problems of poor release ability, slow curing speed, and easy occurrence of oil floating in silicone emulsion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Synthesis of precursor carboxysilane CSi-1 solution
[0037] According to the molar ratio of aliphatic dibasic acid anhydride (DA) and γ-aminopropylsilane (APS) about 1:1, weigh 0.1 mol, about 9.8 g maleic anhydride (MA), 0.1 mol, about 22.14 g γ-amine Propyl triethoxysilane (KH-550) and about 50% by mass of (MA+KH-550), 15.97g propylene glycol dimethyl ether (DMP) solvent, MA was first heated and dissolved into a transparent state, and then Stir, heat and heat up to 70 ° C, then add KH-550 to react for 2 h to obtain a light yellow transparent liquid, that is, the structural formula is (C 2 H 5 O) 3 SiC 3 H 6 The solution of carboxyhydrocarbylsilane (referred to as carboxysilane, CSi-1) of NHCOCH=CHCOOH has a silane content of about 66.67 wt %.
[0038] (2) Preparation of intermediate carboxyl hydrocarbon group / trifluoropropyl modified polysiloxane CFS-1 emulsion
[0039] Press D 4 :D 3 F : The mass ratio of CSi solution is about 30:70:5, take 30.0gD in tur...
Embodiment 2
[0045] (1) Synthesis of precursor carboxysilane CSi-2 solution
[0046] According to the molar ratio of aliphatic dibasic acid anhydride (DA) and γ-aminopropylsilane (APS) about 1:1, weigh 0.1mol, 10.0g succinic anhydride (SA), 0.1mol, about 19.13g γ-aminopropylsilane Methyldiethoxysilane (APMDES) and 100% by mass of (SA+APMDES), about 29.13g propylene glycol methyl ether acetate solvent (PMA), first dissolve SA with solvent to make it transparent, stir and heat up To 125 ℃, add APMDES to stir and react for 4h to obtain a light brown transparent liquid, that is, the structural formula is (C 2 H 5 O) 2 SiMeC 3 H 6 NHCOCH 2 CH 2 The carboxyhydrocarbylsilane of COOH (referred to as carboxysilane, CSi-2) solution has a silane content of about 50 wt%.
[0047] (2) Preparation of intermediate carboxyl hydrocarbon group / trifluoropropyl modified polysiloxane CFS-2 emulsion
[0048] Press D 4 :D 3 F : The mass ratio of CSi-2 solution is about 50:50:20, take 50.0gD in turn 4...
Embodiment 3
[0054] (1) Synthesis of precursor carboxysilane CSi-3 solution
[0055] According to the molar ratio of aliphatic dibasic acid anhydride (DA) and γ-aminopropylsilane (APS) about 1:1, sequentially weigh 0.1mol, about 9.8g MA, 0.1mol, about 17.93g γ-aminopropyltrimethoxy Silane (KH-540) and about 70% by mass of (MA+KH-540), 19.41g of ethylene glycol dimethyl ether solvent, the MA was first heated and dissolved in the solvent into a transparent state, and then stirred and heated to 70 ℃, then add KH-540 to stir and react for 2h to get a light yellow transparent liquid, that is, the structural formula is (CH 3 O) 3 SiC 3 H 6 The carboxyhydrocarbylsilane (abbreviated as carboxysilane, CSi-3) solution of NHCOCH=CHCOOH has a silane content of about 58.82 wt %.
[0056] (2) Preparation of intermediate carboxyl hydrocarbon group / trifluoropropyl modified polysiloxane CFS-3 emulsion
[0057] Press D 4 :D 3 F : The mass ratio of CSi-3 solution is about 40:60:10, take 40.0g D in tu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com