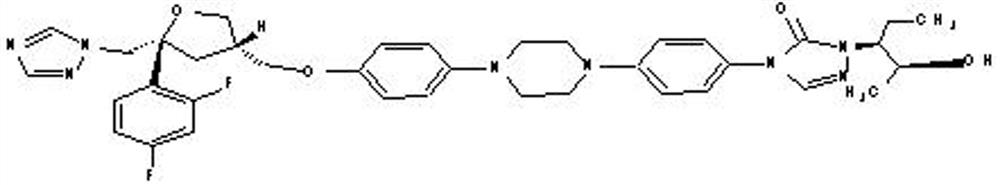
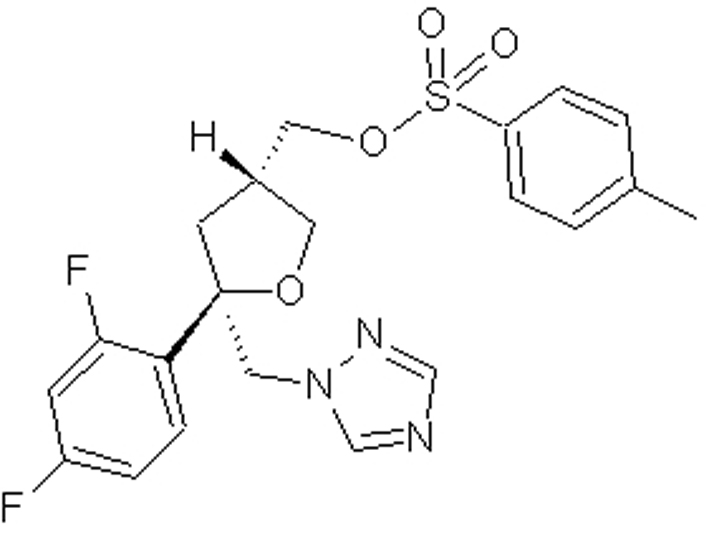
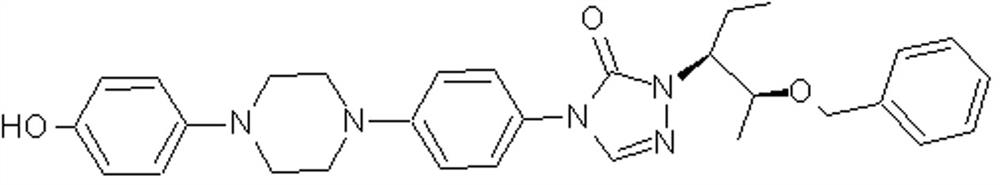
Preparation method of posaconazole main ring intermediate
A technology for the main ring and intermediates of posaconazole, which is applied in the field of medicinal chemistry synthesis, can solve the problems of reducing the reaction conversion rate, high cost, not conforming to the concept of green chemistry, etc., and achieves reducing the generation of by-products, improving yield, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 A kind of preparation method of posaconazole main ring intermediate
[0061] The present embodiment is a preparation method of a posaconazole main ring intermediate, including the following steps performed in sequence:
[0062] Preparation of S1.2-(2,4-difluorophenyl)propan-2-ol
[0063] Take 100.0kg of methyl tertiary butyl ether and place it in a 300L reaction kettle. Under the protective gas nitrogen environment, start the temperature control system to make the temperature of the reaction kettle to -5°C, and add 3mol / L methyl bromide at 85 rpm. 80.0kg of magnesium tetrahydrofuran solution, control the temperature of the system at 3°C, add 20.0kg of 2,4-difluoroacetophenone dropwise, and control the temperature of the system to be 0-5°C during the dropwise addition. After the completion of the dropwise addition, the temperature of the system in the reaction process was controlled to be 15 to 20°C, and the reaction process was monitored by TLC method. Aft...
Embodiment 2-7
[0103] Embodiment 2-7 The preparation method of posaconazole main ring intermediate
[0104] Embodiments 2-7 are respectively a preparation method of a posaconazole main ring intermediate, and their steps are basically the same as those of embodiment 1, and the difference is only in the amount of raw materials and process parameters, and the amount of raw materials in different embodiments is specific. See Table 2 for details, and see Table 3 for various process parameters of different preparation steps.
[0105] Table 2 Raw Material Consumption Table in Examples 2 to 7
[0106]
[0107]
[0108] Table 3 step process parameter table in embodiment 2~7
[0109]
[0110] Example 2-7 A summary table of the yields of 1-[1-(bromomethyl)ethylene]-2,4-difluoro-benzene that finally obtains the target product with 2,4-difluoroacetophenone as a raw material, The specific data are shown in Table 4.
[0111] Table 4 target product yield summary table
[0112]
[0113] As ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com