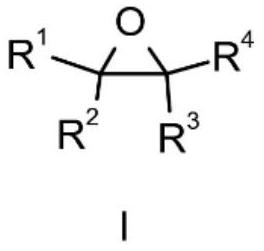
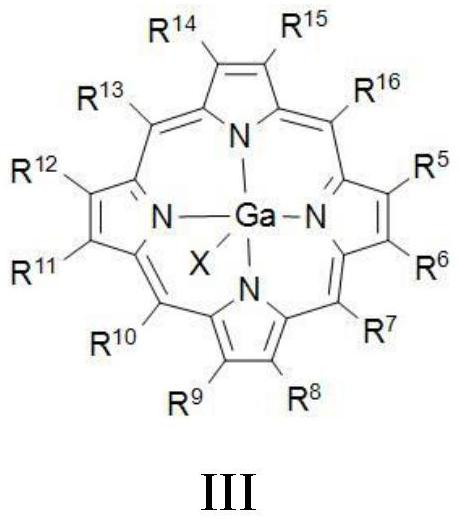
Method for preparing beta-lactone through carbonylation of epoxy compound under catalysis of gallium porphyrin-cobalt carbonyl
An epoxy compound and carbonylation technology, which is applied in the field of β-lactone preparation, can solve the problems of few types of catalysts, sensitivity, and difficulty in catalyst preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0078] Preparation Example: Preparation of Porphyrin Gallium Complex IIIa-IIIe
[0079]
[0080] Porphyrin gallium complexes IIIa-IIIe were synthesized according to the method reported in the literature (Angew.Chem.Int.Ed.2019,58,494).
[0081] Taking compound IIIa as an example, under nitrogen atmosphere, the GaCl 3 (528 mg) was added to a solution of tetraphenylporphyrin IVa (615 mg) in benzonitrile (PhCN). The reaction solution was heated to 150°C and reacted for 20 hours. During the reaction, the solution gradually changed from green to dark purple. The reaction was cooled to room temperature and the solvent was removed under reduced pressure. The residue was purified by neutral alumina column chromatography (CH 2 Cl 2 / MeOH). The eluate was washed with 1M HCl, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain the porphyrin gallium complex IIIa. Yield 82%, purple solid.
[0082] Tetraphenylporphyrin IVa is replaced ...
Embodiment 1
[0083] Example 1: Solvent p-tetraphenylporphyrin gallium / Co 2 (CO) 8 The effect of synergistically catalyzed propylene oxide carbonylation
[0084]
[0085] Into a 125mL autoclave, add tetraphenylporphyrin gallium complex IIIa (0.050mmol), Co 2 (CO) 8 (25.6 mg, 0.075 mmol), solvent (10 mL), propylene oxide (5.81 g, 100.0 mmol). After sealing the autoclave, replace N 2 Three times, 20 atm of carbon monoxide was charged into the autoclave, and the temperature was raised to 80° C. and stirred for 16 hours. After cooling the autoclave in an ice-water bath for 1.5 hours, the excess carbon monoxide was slowly released. 1.5 mL of internal standard n-tridecane was added to the autoclave, and the mixture was stirred uniformly. Use gas chromatography method (using the standard curve method, that is, take n-tridecane as the internal standard, make a standard curve for β-butyrolactone on the gas chromatography with the peak area ratio of n-tridecane, by measuring the reaction sys...
Embodiment 2
[0089] Example 2: Reaction temperature and pressure p-tetraphenylporphyrin gallium / Co 2 (CO) 8 The effect of synergistically catalyzed propylene oxide carbonylation
[0090]
[0091] Into a 125mL autoclave, add tetraphenylporphyrin gallium complex IIIa (0.050mmol), Co 2 (CO) 8 (25.6 mg, 0.075 mmol), tetrahydrofuran (10 mL), propylene oxide (5.81 g, 100.0 mmol). After the autoclave was sealed, nitrogen was replaced three times, carbon monoxide was charged into the autoclave to the required pressure, and the reaction was stirred at a predetermined temperature for 16 hours. After cooling the autoclave in an ice-water bath for 1.5 hours, the excess carbon monoxide was slowly released. 1.5 mL of internal standard n-tridecane was added to the autoclave, and the mixture was stirred uniformly. Use gas chromatography method (using the standard curve method, that is, take n-tridecane as the internal standard, make a standard curve for β-butyrolactone on the gas chromatography wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com