Green synthesis method of cidofovir
A technology of green synthesis and synthesis process, applied in the direction of organic chemistry methods, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., to achieve the effects of mild reaction conditions, ee value maintenance, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
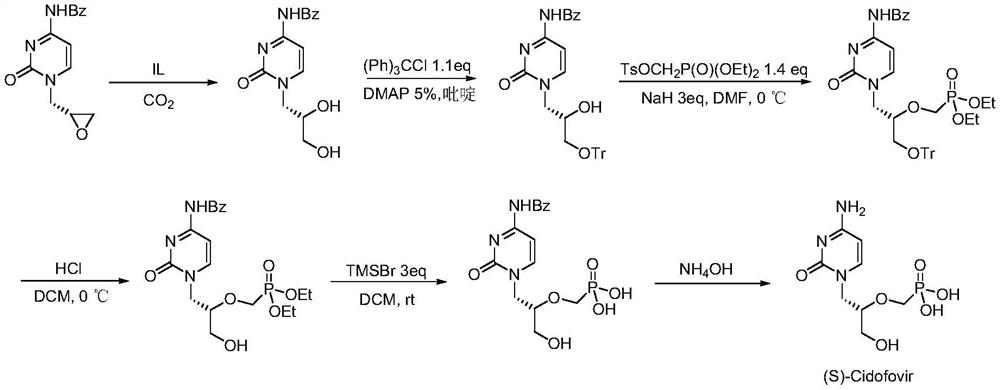
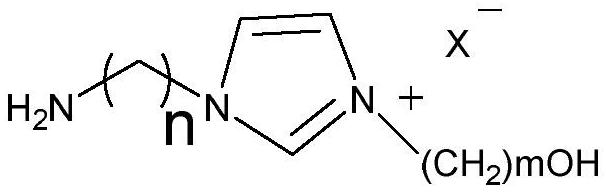
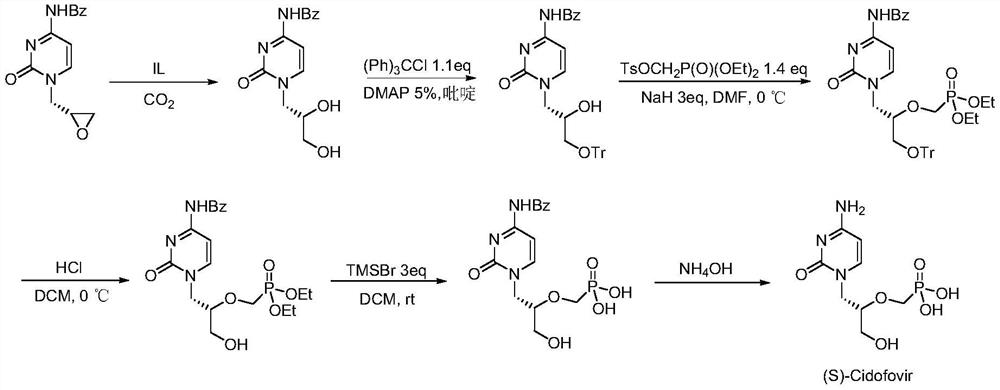
[0015] N4-benzoylcytosine epoxy compound (1mmol) (ee value 99%), ionic liquid catalyst (1mmol) and water (10mmol) were mixed uniformly and added to the autoclave to replace CO 2 gas three times, then put the autoclave into a heating stirrer with a temperature of 60 ° C, and feed CO of 2 MPa 2 gas, after 24 hours of continuous reaction, the autoclave was cooled and excess gas was released, then dichloromethane was added to separate the product, and the chiral N4-benzoylcytosine diol compound was obtained by column chromatography, and its yield was 92%, ee value 99%. The structural formula of the ionic liquid catalyst used in the synthesis process is where m=4, n=4, and X=Br.
Embodiment 2
[0017] N4-benzoylcytosine epoxy compound (1mmol) (ee value 99%), ionic liquid catalyst (1mmol) and water (35mmol) were mixed uniformly and added to the autoclave to replace CO. 2 gas three times, then put the autoclave into a heating stirrer with a temperature of 80 ° C, and feed CO 2 of 2 MPa. 2 After 18 hours of continuous reaction, the autoclave was cooled and excess gas was released, and then dichloromethane was added to separate the product and the ionic liquid catalyst. The ionic liquid catalyst was washed and dried for next use, and the product was obtained by column chromatography. The chiral N4-benzoylcytosine diol compound has a yield of 84% and an ee value of 99%. The structural formula of the ionic liquid catalyst used in the synthesis process is where m=2, n=3, and X=OH.
Embodiment 3
[0019] N4-benzoylcytosine epoxy compound (1mmol) (ee value 99%), ionic liquid catalyst (1mmol) and water (45mmol) were mixed and added to the autoclave to replace CO. 2 gas three times, then put the autoclave into a heating stirrer with a temperature of 70 ° C, and feed 2 MPa of CO 2 gas, after 12 hours of continuous reaction, the autoclave was cooled and excess gas was released, and then dichloromethane was added to separate the product and the ionic liquid catalyst. The ionic liquid catalyst was washed and dried for next use, and the product was obtained by column chromatography. The chiral N4-benzoylcytosine diol compound has a yield of 86% and an ee value of 99%. The structural formula of the ionic liquid catalyst used in the synthesis process is where m=6, n=2, and X=Br.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com