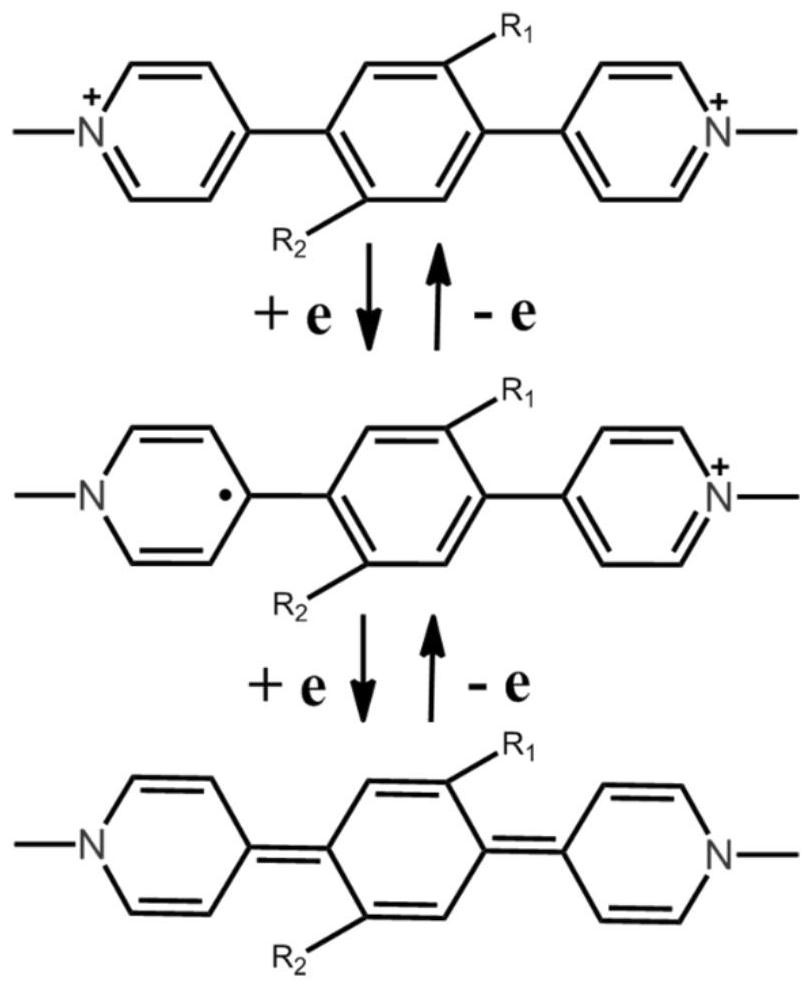
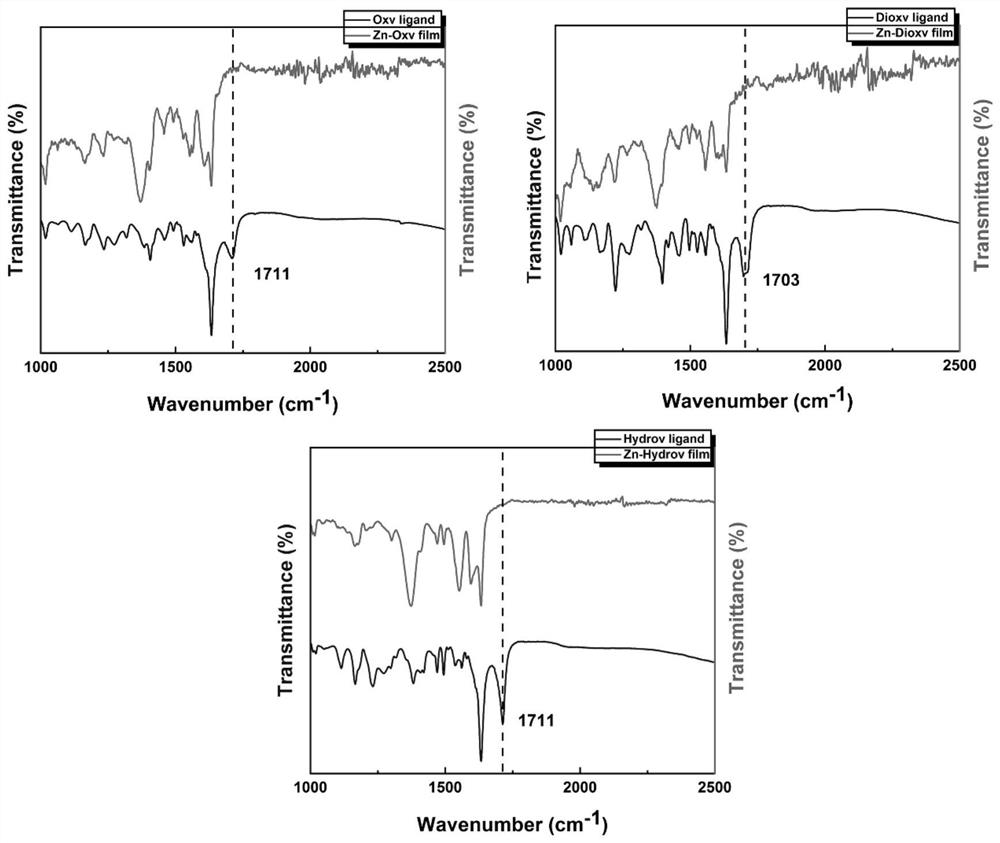
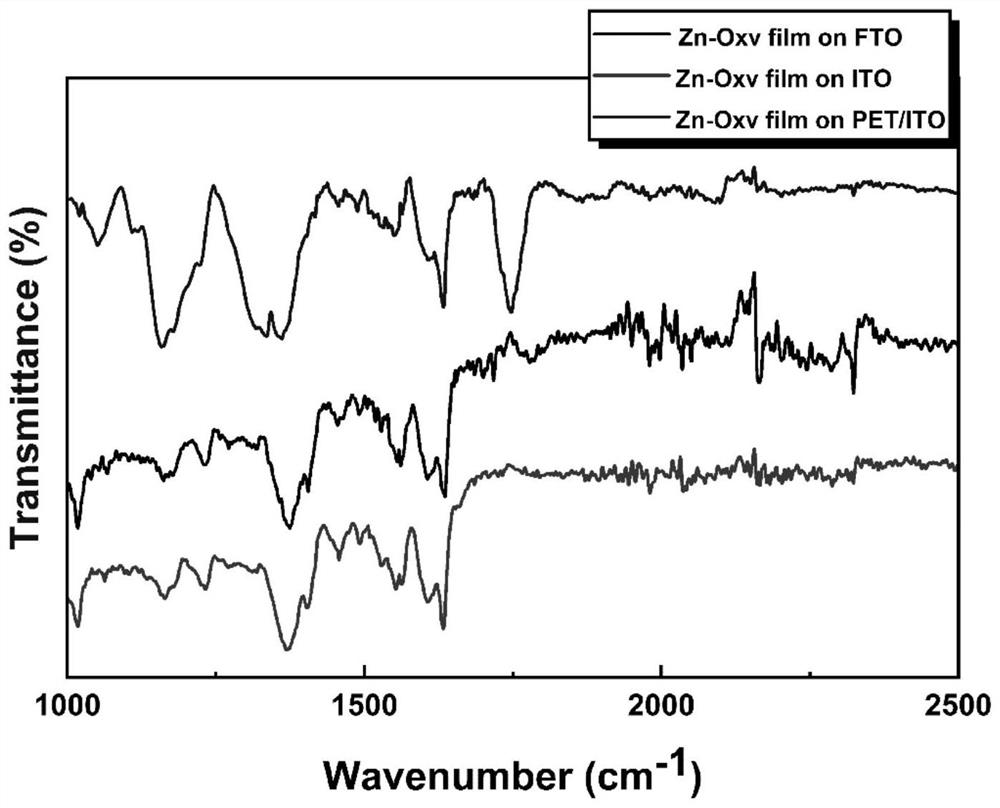
Preparation method and application of viologen complex film
A technology of complexes and viologens, which is applied in the field of preparation of viologen complex films, can solve the problems of difficulty in obtaining complex films, long time consumption, and little research on electrochromism of viologen complexes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a preparation method of a viologen complex film, comprising:
[0031] A three-electrode system was used to reduce the viologen ligand monomer on the surface of the working electrode to obtain a viologen complex film;
[0032] The electrodeposition solution used in the reduction process includes: a viologen ligand monomer, a metal source, an electrolyte and a solvent.
[0033] In the present invention, the three-electrode system preferably includes: a working electrode, a reference electrode and a counter electrode.
[0034] In the present invention, the working electrode is preferably a conductive substrate, more preferably selected from ITO (indium tin oxide conductive glass), FTO (fluorine-doped SnO) 2 Conductive glass), PET / ITO (indium tin oxide coated PET conductive film).
[0035] In the present invention, the working electrode is preferably washed, dried and trimmed.
[0036] In the present invention, the washing method is preferably ultr...
Embodiment 1
[0079] Example 1 Synthesis of viologen derivative ligand (Oxv)
[0080] The preparation of Oxv ligand was carried out according to the following route:
[0081]
[0082] Into a 250 mL round-bottomed flask were added 4-pyridineboronic acid (1.0 g, 8.1 mmol), 2,5-dibromoanisole (1.2 g, 4.5 mmol), potassium carbonate (1.2 g, 8.6 mmol), tetrakistriphenyl Phosphine palladium (0.28g, 0.20mmol) and a magnetic stirring bar, the bottle was evacuated; 50mL of a mixed solvent of dioxane and water 4:1 was injected into the round-bottomed flask, and the reaction system was protected with argon gas. The reaction system was stirred at 90°C for 48h to carry out the reaction; after the reaction, the obtained reaction product was cooled to room temperature, the solvent was removed by rotary evaporation at 50°C, dissolved in chloroform, the organic phase was extracted, and then the solvent was rotated to dryness to obtain a crude product ; Purify the crude product with ethyl acetate column c...
Embodiment 2
[0085] Example 2 Synthesis of Violet Derivative Ligand (Dioxv)
[0086] The preparation of Dioxv ligands was carried out according to the following route:
[0087]
[0088] The ligand was prepared according to the method of Example 1, and the difference from Example 1 was that 2,5-dibromodimethoxybenzene (1.3 g, 4.5 mmol) was used.
[0089] The product prepared by embodiment 2 is carried out hydrogen nuclear magnetic resonance spectrum detection, and the detection result is such as Image 6 b, the data are: 1HNMR (300MHz, DMSO-d6, ppm): δ9.314-9.264(d,4H), 8.517-8.469(d,4H), 8.055-8.011(d,4H), 7.731-7.687 (d, 4H), 7.538-7.498 (S, 2H), 6.017-5.951 (S, 4H), 3.948-3.900 (S, 6H); it can be seen that the method of Example 2 can prepare the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com