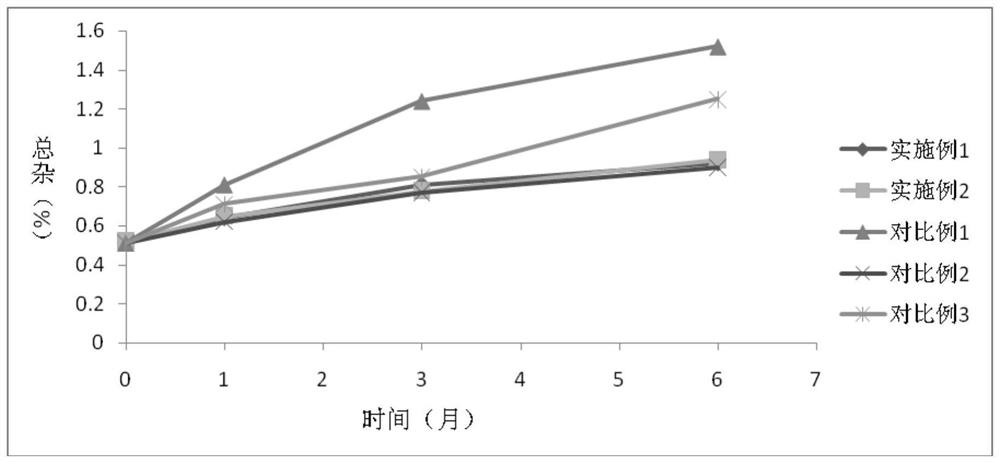
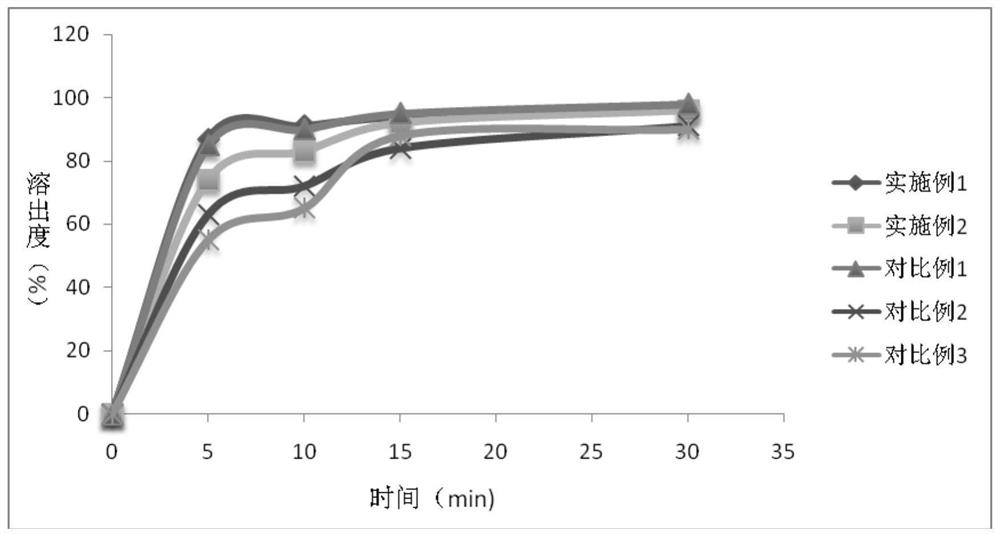
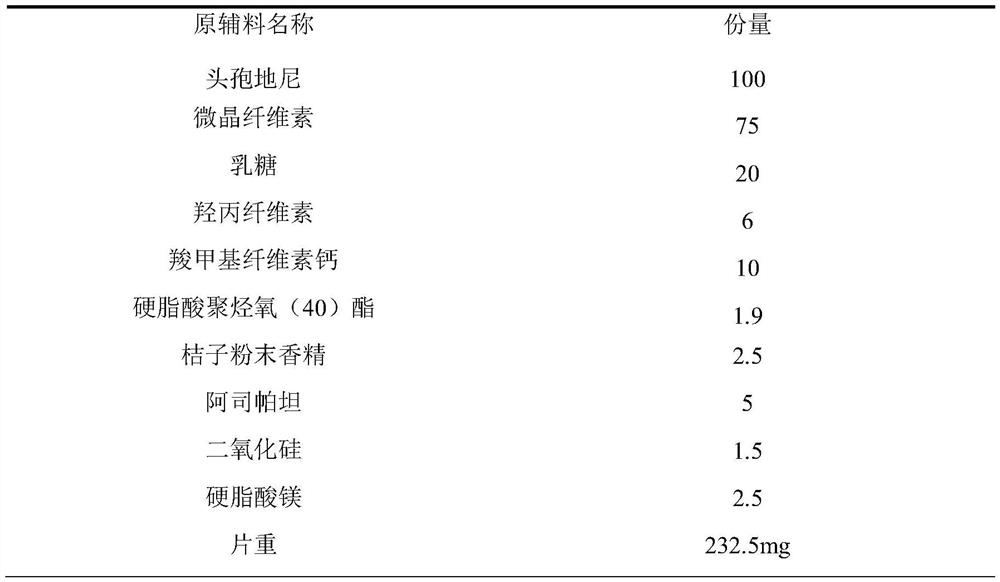
Cefdinir dispersible tablet pharmaceutical composition and preparation process thereof
A technology of cefdinir and composition, applied in the field of pharmaceutical composition of cefdinir dispersible tablet and preparation field thereof, can solve the problems of poor stability, low bioavailability, slow disintegration and dissolution, etc., and achieve good compatibility , good hydrophilicity, good disintegration time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation Example
[0022]
[0023] Preparation Process
[0024] 1) Weigh the appropriate proportion of cefdinir, microcrystalline cellulose, lactose, hydroxypropyl cellulose, calcium carboxymethyl cellulose, polyoxyl (40) stearate, aspartame, and orange powder flavor and mix well , Use a dry granulator for granulation, the granulation pressure is 40-60bar, and the number of whole grains is 14.
[0025] 2) Put the recipe amounts of silicon dioxide, magnesium stearate and the above particles into a three-dimensional mixer for total mixing.
[0026] 3) Press the tablet with a 9mm circular shallow concave punch.
Embodiment 2
[0027] Example 2: Preparation Example
[0028]
[0029] Preparation Process
[0030] 1) Weigh the appropriate proportion of cefdinir, microcrystalline cellulose, lactose, hydroxypropyl cellulose, calcium carboxymethyl cellulose, polyoxyl (40) stearate, aspartame, and orange powder flavor and mix well , Use a dry granulator for granulation, the granulation pressure is 40-60bar, and the number of whole grains is 14.
[0031] 2) Put the recipe amounts of silicon dioxide, magnesium stearate and the above particles into a three-dimensional mixer for total mixing.
[0032] 3) Press the tablet with a 9mm circular shallow concave punch.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com