Tinidazole tablet and preparation method thereof
A technology of tinidazole tablets and nidazole tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of poor smoothness of tinidazole tablet compression process and easy occurrence of Grinding, low production efficiency and other problems, to avoid the growth of genotoxic impurities, reduce production costs and energy consumption, and overcome the effects of poor process fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Tinidazole tablet of the present invention adopts dry granulation process to prepare, and its preparation method specifically comprises the following steps:
[0030] S1. Weigh raw materials: Weigh raw materials and auxiliary materials according to the prescription.
[0031] S2. Premixing: Put the prescribed amount of tinidazole, filler, part of the disintegrant, glidant, and part of the binder into the mixer and mix for 20-40 minutes, and mix evenly.
[0032] S3. Granulation: adopt dry granulation process to granulate, the conveyor speed is 80-200rpm, the spacing between the pressing wheels is 0.2mm, the rotating speed of the pressing wheels is 20-40rpm, and the sizing screen is 18 mesh.
[0033] S4. Total mixing: The granules are mixed with the remaining disintegrant, remaining binder and lubricant, and the mixing time is 10-20 minutes.
[0034] S5. Feed the total mixed granules into the tablet press, control the hardness to 8.0-17.0kg, the tablet weight difference is...
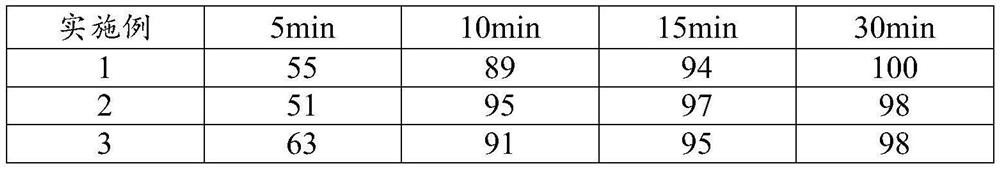
Embodiment 1~6
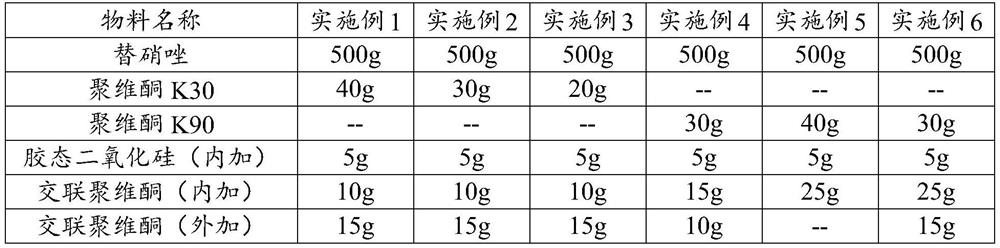
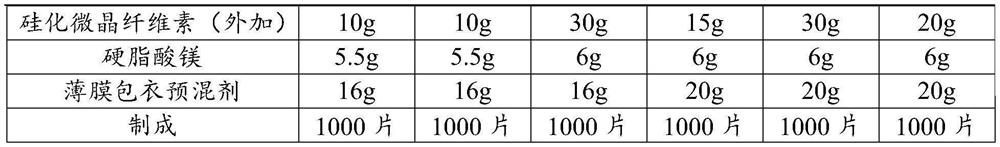
[0047] The formula of embodiment 1~6 is as shown in table 3:
[0048] The formula of table 3 embodiment 1~6
[0049]
[0050]
[0051] Wherein, the preparation method of embodiment 1~6 is as follows:
[0052] S1. Weigh raw materials: Weigh raw materials and auxiliary materials according to the prescription.
[0053] S2. Premixing: Put the prescribed amount of tinidazole, filler, povidone, colloidal silicon dioxide, and crospovidone (internal addition) into the mixer and mix for 20-40 minutes, and mix well.
[0054] S3. Granulation: Dry granulation process is used for granulation, and the specific process parameters are as follows: conveyor speed 80-200rpm, pressure wheel spacing 0.2mm, pressure wheel speed 20-40rpm, granulation screen 18 mesh.
[0055]S4, total blending: the granules after granulation and crospovidone (additional), silicified microcrystalline cellulose (additional) and magnesium stearate are put into the mixer and mixed, the mixing speed is 10rpm, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com