Butorinol tartrate crystal form I as well as preparation method and application of butorinol tartrate crystal form I
A technology of butorphanol and tartaric acid, which is applied in the field of drug crystal forms, can solve problems such as undiscovered butorphanol tartrate new crystal forms, changes in product crystal forms or purity, and reduced crystallization yields, and achieve product quality risks Low concentration, good crystal stability, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 0.79Kg (1.0L) of ethanol and butorphanol free base (100g, 0.306mol) to the reaction kettle A, stir, and heat up to reflux to dissolve. Add 0.21Kg of purified water in the reactor B, add D-tartaric acid 68.8g (1.5eq.), add the D-tartaric acid solution in the reactor B to the clear butorphanol solution, add, and slowly cool down to 30 Below ℃, continue to stir for 4-6 h, filter, and dry the filter cake under reduced pressure to constant weight to obtain 121 g of crystalline solid, yield: 83%, HPLC purity 99.98%, total impurities 0.02%.
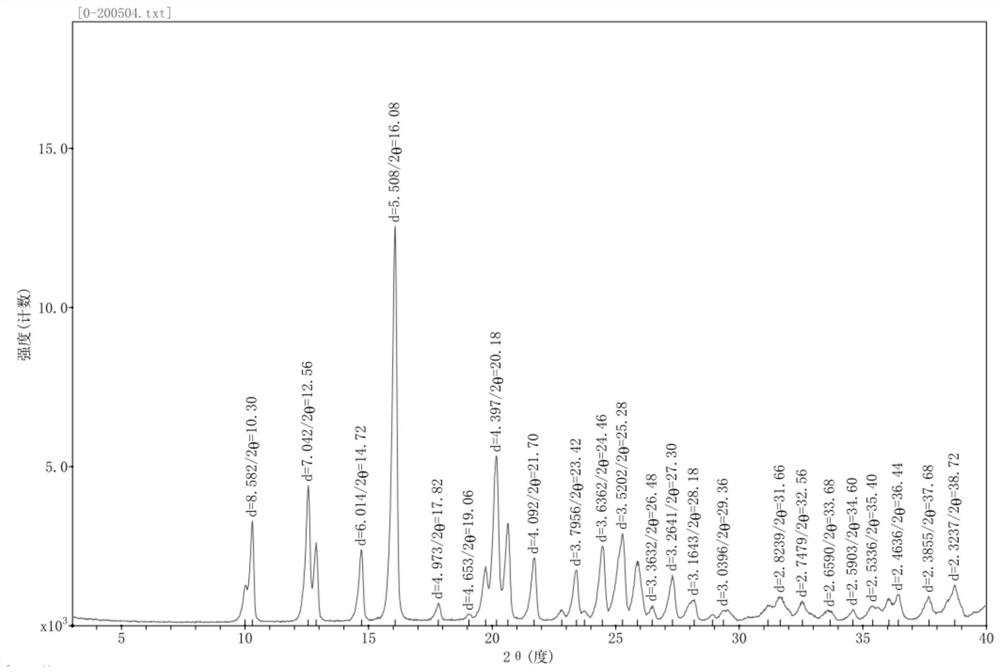
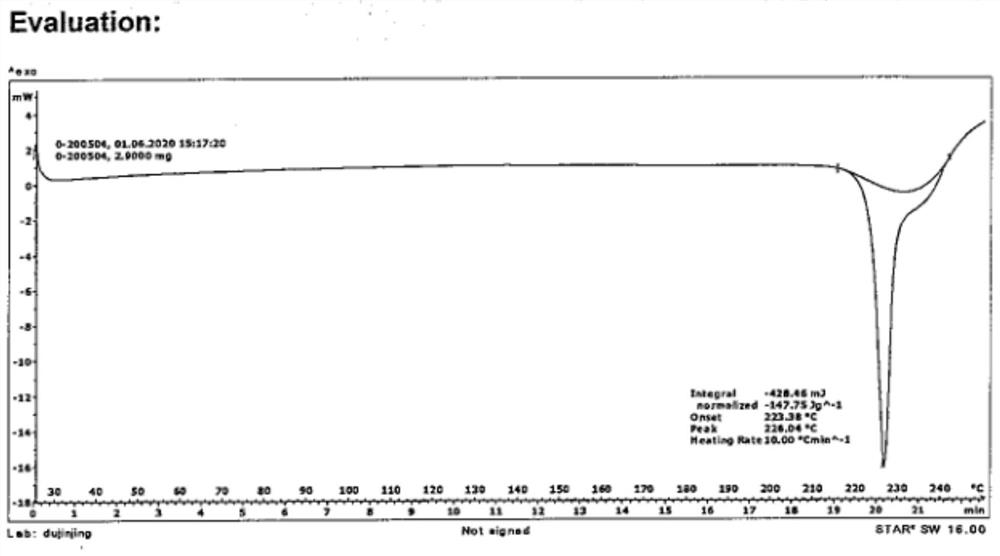
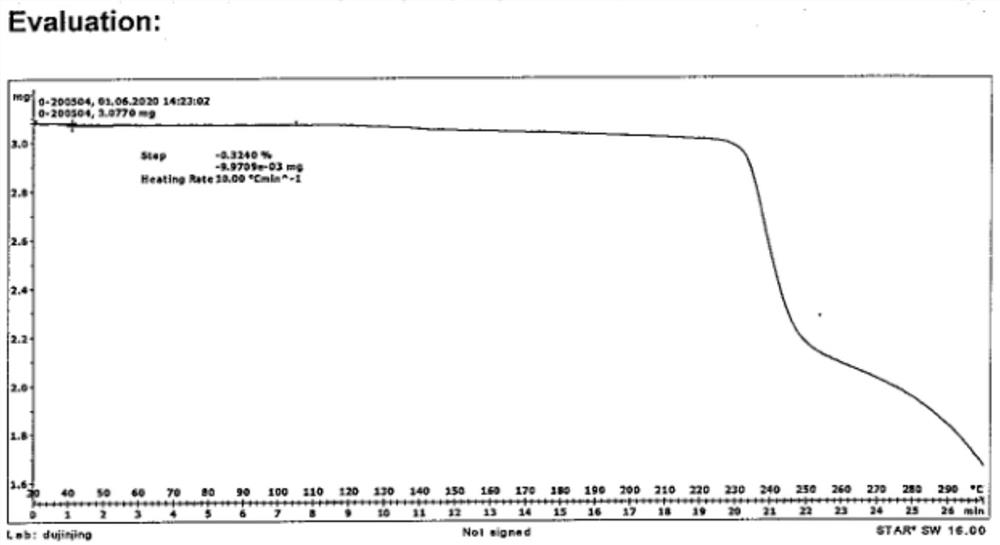
[0047] The powder diffraction pattern of the crystalline sample is shown in figure 1 , the data are shown in Table 1, DSC has a characteristic absorption peak at 223.38 ° C, TGA shows that the crystalline sample begins to decompose and lose weight when heated to 223.38 ° C, the water content of the crystal form is measured by KF method, and the water content is 0.16%.
[0048]Table 1:
[0049]
[0050]
Embodiment 2、 Embodiment 3
[0052] Add butorphanol free base (0.5 g) into the reaction flask, add an appropriate amount of solvent according to Table 2 below, heat up to reflux and stir to dissolve. Quantitatively weigh 0.25 g of D-tartaric acid, add 0.75 ml of purified water and stir to dissolve, slowly add the tartaric acid aqueous solution to the butorphanol free base solution, and slowly cool down to 0 °C after the dropwise addition is completed, stir and crystallize for 4-6 hours, There is solid precipitation, centrifuge, the obtained solid is dried to constant weight at 40°C, yield: 84%, HPLC purity 99.98%, total impurities 0.02%, the obtained sample is measured by XRD, see Figure 5 .
[0053] Table 2:
[0054] solvent Solvent dosage Crystal form Example 2 methanol 2.5mL Form I Example 3 isopropyl alcohol 5.0mL Form I
Embodiment 4、5、6
[0056] The butorphanol free base (0.5 g) was added to the reaction flask, an appropriate amount of ethanol was added according to Table 3 below, and the temperature was raised and refluxed and stirred until it was dissolved. Quantitatively weigh 0.25 g of D-tartaric acid, add 0.75 ml of purified water and stir to dissolve it, slowly add the tartaric acid aqueous solution to the ethanol solution of butorphanol free base, slowly cool down to 0 °C after the dropwise addition, stir and crystallize 4- 6h, a large amount of solid was precipitated, centrifuged, and the obtained solid was dried at 40°C to constant weight, yield: 84%, HPLC purity 99.97%, total impurities 0.03%, the obtained sample was measured by XRD, see attached Image 6 .
[0057] table 3:
[0058] Solvent dosage Crystal form Example 4 2.5ml Form I Example 5 5.0ml Form I Example 6 7.5ml Form I
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com