Lacosamide oral solution and preparation method thereof
An oral solution, lacosamide technology, applied in the direction of pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problem of the safety of synthetic sweeteners, poor solution stability, Easy to corrupt and other problems, achieve good taste masking effect and stability, simple preparation method, and reduce the effect of contact time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
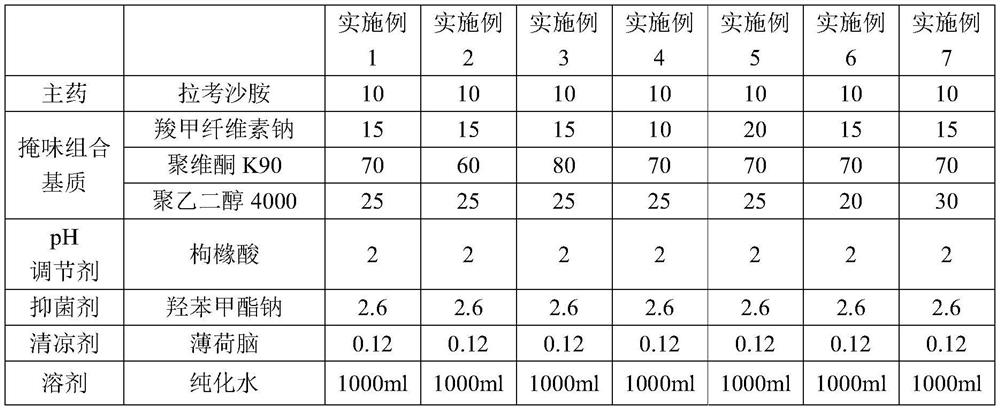
[0037] The lacosamide oral solution provided by the present invention, its formula is shown in Table 1.
[0038] The formula of the lacosamide oral solution in the embodiment of table 1
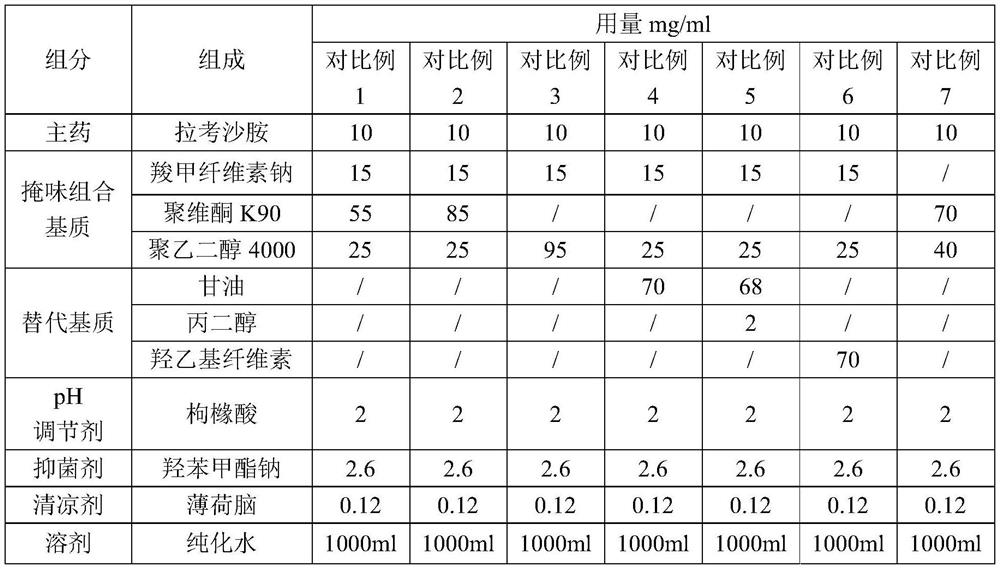
[0039]
[0040]
[0041] The preparation method of oral solution in embodiment 1-7 is as follows:
[0042] (1) the total amount is 60% of purified water in the dosing tank, then add bacteriostatic agent and pH regulator, and stir until dissolved;
[0043] (2) heating the obtained mixed solution in step (1) to 60°C, adding active component lacosamide to it, and stirring until dissolved;
[0044] (3) slowly add sodium carboxymethyl cellulose, povidone K90 and polyethylene glycol 4000, and stir until dissolved;
[0045] (4) Add the cooling agent and stir until it dissolves, then add the remaining purified water to make the volume to 1000ml, filter, and fill to get it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com