Naphthyl urea-piperazine compound as well as preparation method and application thereof
A compound, naphthylurea technology, applied in the field of tumor targeted therapy, can solve the problem of unmet demand for JAKs/STAT3 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis of Compounds
[0049]
[0050] ID210916B-1:
[0051] ID210917B-1:
[0052] ID210918B-1:
[0053] ID210919B-1:
[0054] ID211130C-1:
[0055] ID211203B-1:
[0056] IY211214C-1:
[0057] IY211228B-1:
[0058] IY220302B-1:
[0059] IY220209A-1:
[0060] IY220313A-1:
[0061] IY220219A-1:
[0062] ID210928B-1:
[0063] ID210929B-1:
[0064] IY220319A-1:
[0065] ID211008B-1:
[0066] ID211009B-1: R 3 =H;
[0067] ID211010B-1: R 3 =CH 3 ;
[0068] ID211116B-1:
[0069] ID211012B-1:
[0070] The specific synthesis method, taking compound ID210916B-1 as an example, the structural formulas are as follows:
[0071]
[0072] Compound ID210916B-1 is named tert-butyl 4-(2-(4-(((4-(3-(pyridin-4-ylmethyl)ureido)naphthalen-1-yl)oxy)methyl)phenoxy)ethyl)piperazine -1-carboxylate,
[0073] Its synthetic route is as follows:
[0074]
[0075] Step 1. tert-butyl 4-(2-(4-formylphenoxy)ethyl)pipera...
Embodiment 2
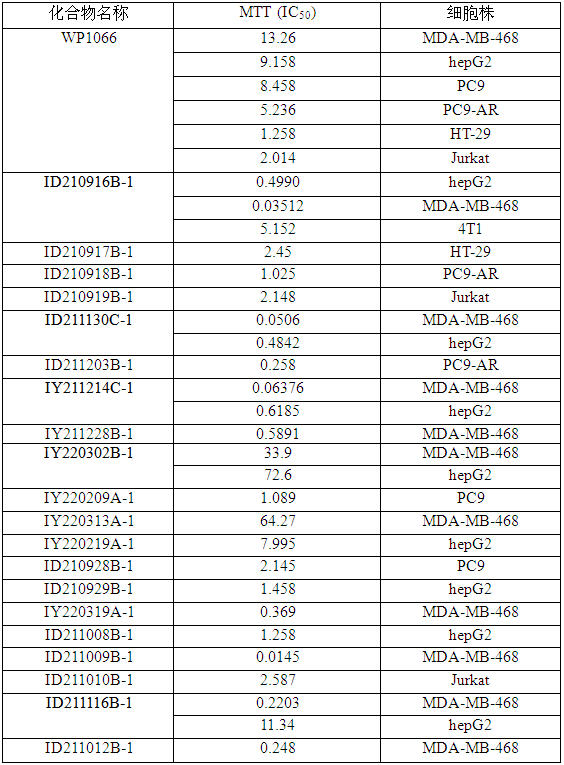
[0121] Example 2: ID210916B-1, ID210917B-1, ID210918B-1, ID210919B-1, D211130C-1, ID211203B-1, IY211214C-1, IY211228B-1, IY220302B-1, IY220209A-1, IY22A-1, IY222031 1, ID210928B-1, ID210929B-1, IY220319A-1, ID211008B-1, ID211009B-1, ID211010B-1, ID211116B-1 and ID211012B-1, etc. on the proliferation inhibition of breast cancer and liver cancer cells
[0122] Collect tumor cells in logarithmic growth phase respectively, adjust the concentration of cell suspension to 5 × 10 4 cells / mL, added to a 96-well cell culture plate with a volume of 100ul per well. With DMSO as solvent control, WP1066 (Chinese name: (2E)-3-(6-bromo-2-pyridyl)-2-cyano-N-[(1S)-1-phenylethyl]-2- Acrylamide, CAS: 857064-38-1, the structure is ) as a positive control, the novel naphthalene urea-piperazine compounds ID210916B-1, ID210917B-1, ID210918B-1, etc. of the present invention were diluted with DMSO and added to the culture well, so that the final concentrations of the compounds in the system were 0.1...
Embodiment 3
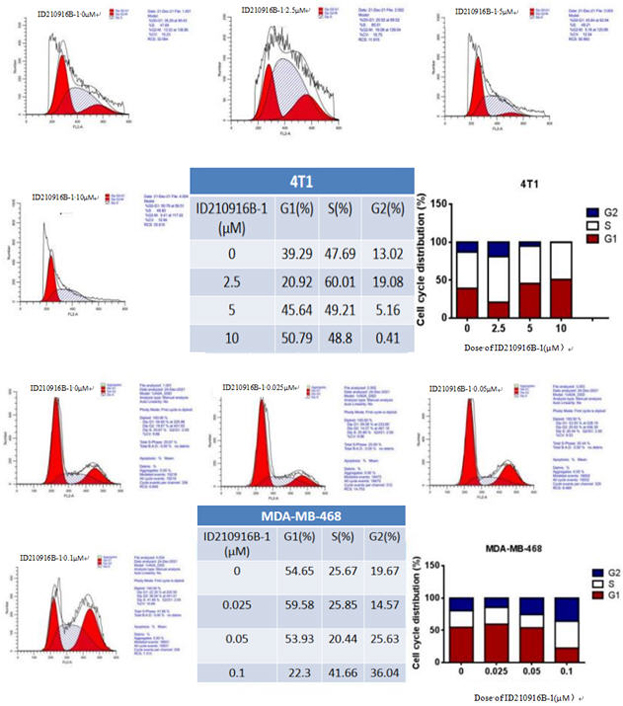
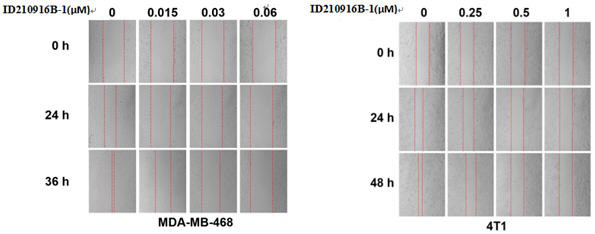
[0124] Example 3: Effect of ID210916B-1 on induction of TNBC cell cycle arrest
[0125] MDA-MB-468 or 4T1 cells in logarithmic growth phase were taken, digested and centrifuged to make single cell suspension. After counting, cells were plated into a 12-well plate, and both cells were seeded at 2 × 10 per well. 5 Cells were plated in 3 wells for parallel control. 16h after plating, the cells were treated with compound. Using DMSO as the compound solvent, the final concentration of compound ID210916B-1 in 4T1 cell suspension was 0, 2.5, 5 and 10 μM, respectively, and the final concentration of compound ID210916B-1 in MDA-MB-468 cell suspension was 0, 0.025, respectively , 0.05 and 0.1 μM. 48h after dosing, each empty cell was digested with trypsin, resuspended and counted, and the cell concentration in each well was adjusted to 5×10 5 indivual. After digestion, centrifuge and discard the supernatant, then wash the cells twice with PBS (2000 rpm, centrifugation for 5 min), t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com