Multifunctional antibody composition
A composition and multi-functional technology, applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., can solve the problems of light and heavy chain mismatch, complex structure, etc., and achieve good pharmacodynamics and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Acquisition and Optimization of Nucleotide Sequences
[0065] The amino acid sequence information of the light chain and heavy chain of the multifunctional antibody is selected from the public or self-developed PD-1 target monoclonal antibody sequence information, and the variable region and constant region information of the sequence are obtained by analysis. The native IL-15 sequence or the IL-15 variant sequence is inserted into the amino acid sequence of one heavy chain, and the IL-15 receptor sequence, preferably the IL-15RαSushi sequence, is inserted in the corresponding position of the other heavy chain. According to needs, adjust the Fc of the antibody amino acid sequence to other IgG types, such as IgG1, etc., and further design the desired form of amino acid mutation in each heavy chain, thereby obtaining the amino acid sequence of the target antibody, respectively:
[0066] The first heavy chain is SEQ ID NO:1, the second heavy chain is SEQ ID NO:3,...
Embodiment 2
[0070] Example 2 Gene synthesis and construction of expression vector
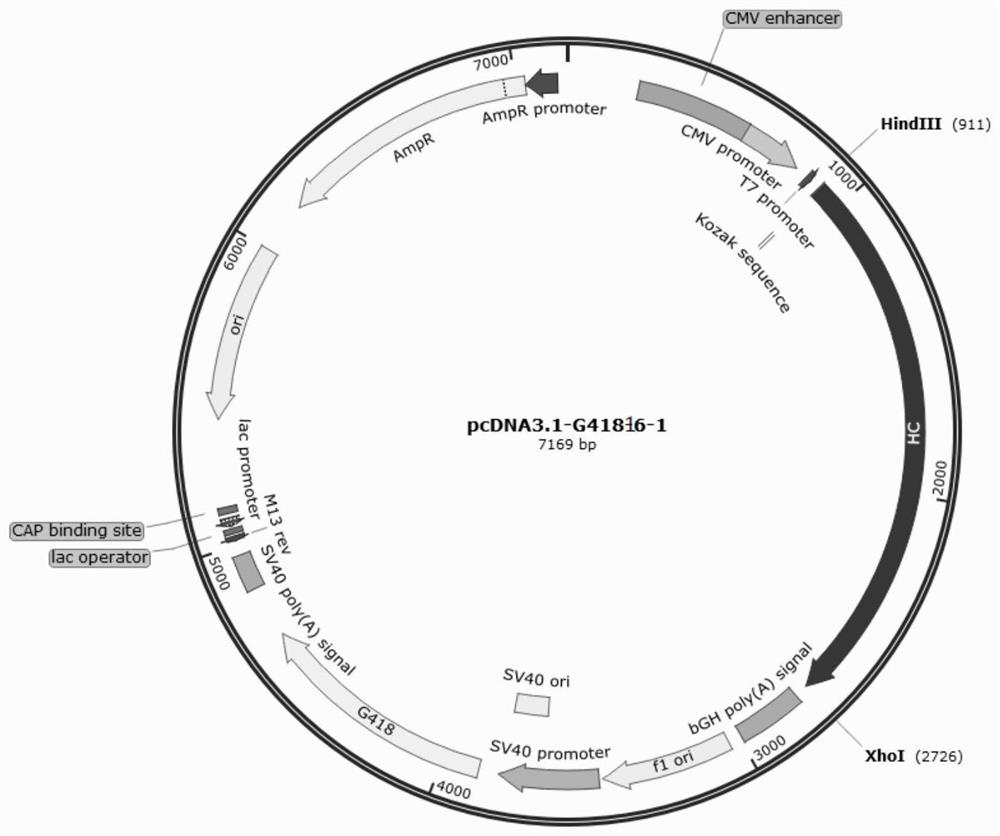
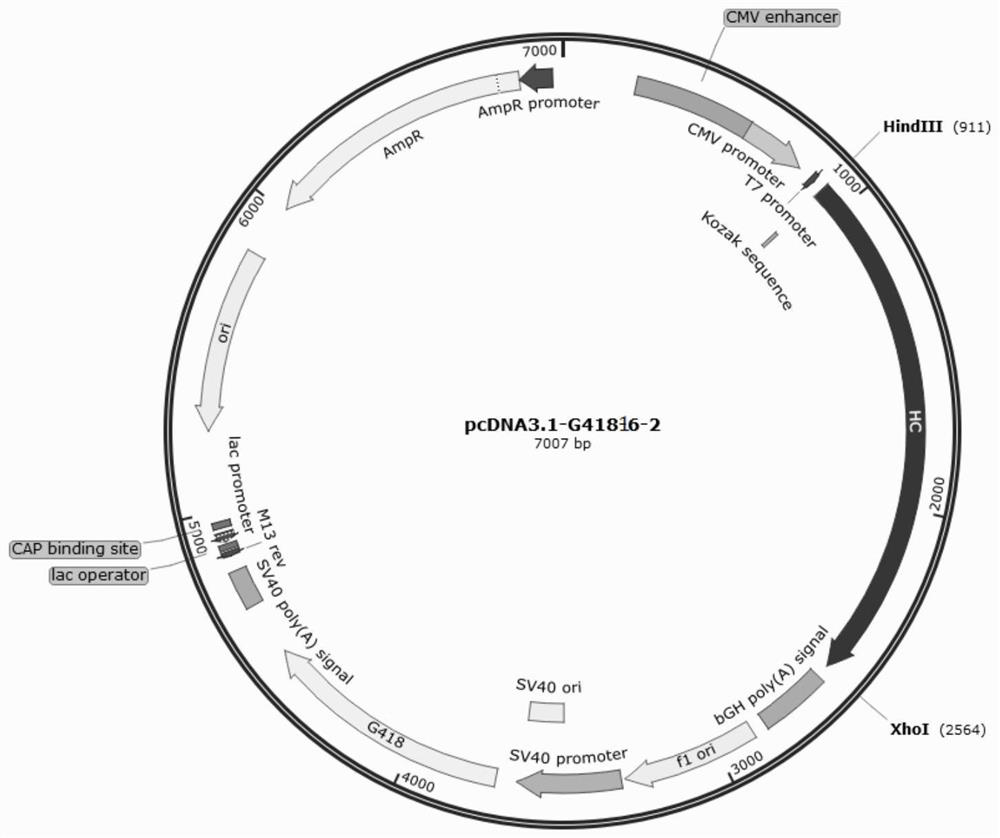
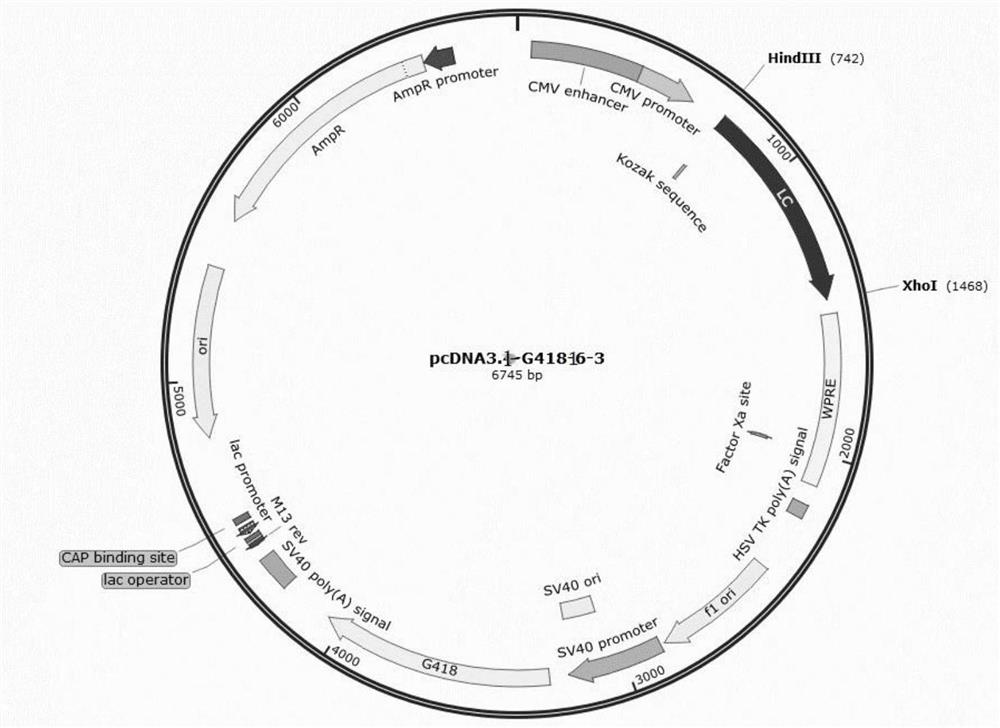
[0071] The pcDNA3.1-G418 vector was used as a dedicated vector for expressing the light and heavy chains of the multifunctional antibody. The pcDNA3.1-G418 vector contains the promoter CMVPromoter used for the heavy chain, the eukaryotic selection marker G418 tag and the prokaryotic selection tag Ampicilline. The nucleotide sequence of the light chain and heavy chain of the antibody of the multifunctional antibody obtained by gene synthesis was double digested with HindIII and XhoI, and the DNA ligase was used for enzymatic ligation after recovery, and transformed into E. coli competent Cell DH5α, select positive clones and carry out plasmid extraction and enzyme digestion verification to obtain recombinant plasmids containing the full-length first heavy chain, second heavy chain, first light chain and second light chain of the multifunctional antibody, respectively pcDNA3.1-G418-16-1, pcDNA3.1-G418-16-2 ...
Embodiment 3
[0072] Example 3 Plasmid extraction
[0073]According to the method described in "Molecular Cloning Experiment Guide" (Science Press, 2002), the recombinant plasmids containing the above target genes were transformed into E. coli competent cells DH5α, and the transformed bacteria were spread on 100 μg / ml ampicillin-containing Cultivate on LB plate, select plasmid clones and culture in liquid LB medium, shake bacteria at 260 rpm for 14 hours, extract plasmids with endotoxin-free plasmid extraction kit, dissolve with sterile water and measure the concentration with nucleic acid protein quantifier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com