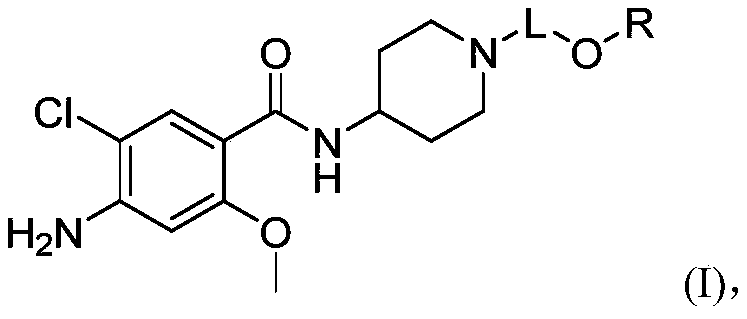
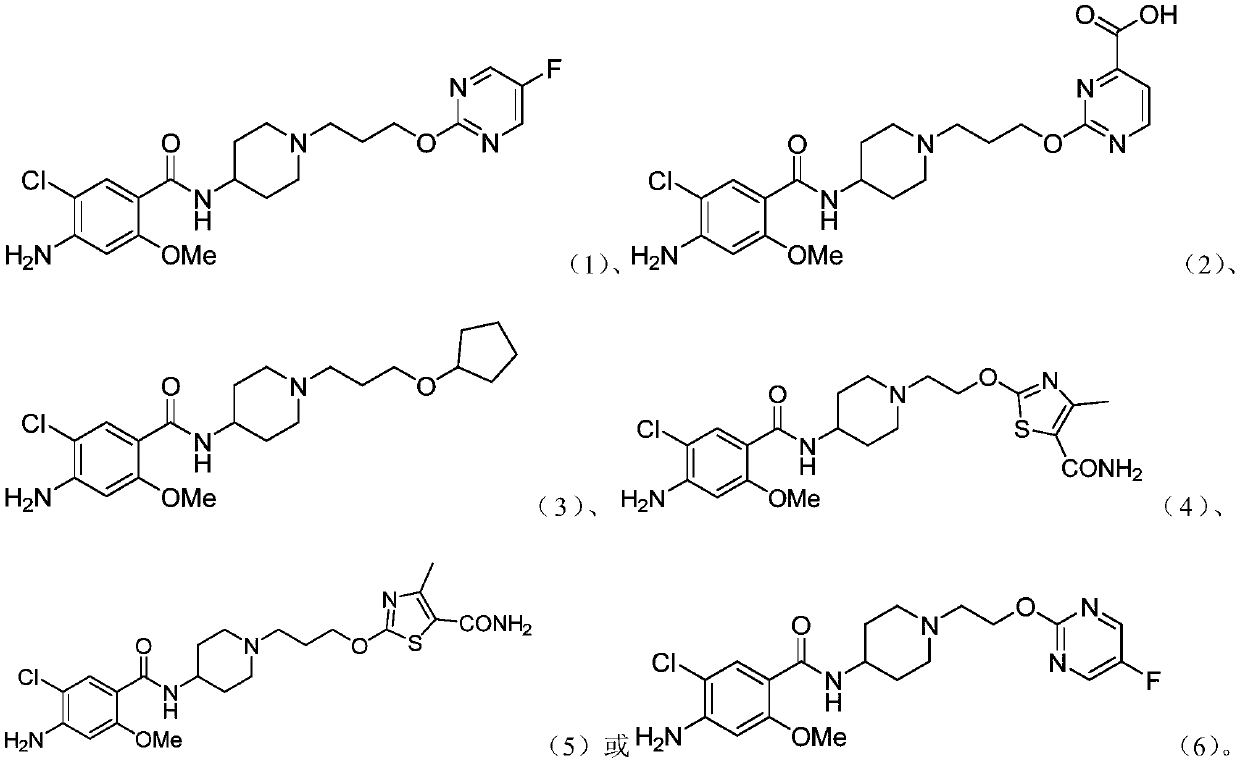
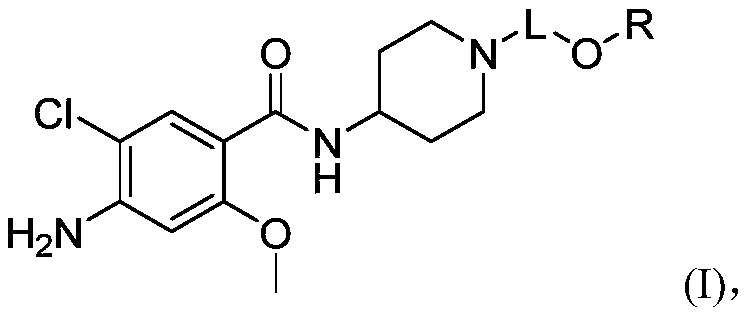
Substituted benzamide derivative and application thereof
A technology of drugs and compounds, applied in the field of constipation-type irritable bowel syndrome, can solve problems such as poor clinical treatment effect, and achieve the effects of good pharmacokinetic properties, good brain/plasma ratio, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Example 1 4-amino-5-chloro-N-(1-(3-((5-fluoropyrimidin-2-yl)oxy)propyl)piperidin-4-yl)-2-methoxybenzene Synthesis of formamide
[0189]
[0190] Step 1) Synthesis of (1-(3-hydroxypropyl) piperidin-4-yl) tert-butyl carbamate
[0191] In a 100mL single-necked bottle, add tert-butyl N-(4-piperidinyl)carbamate (2g, 9.99mmol), 3-bromopropan-1-ol (2.08g, 14.98mmol), potassium carbonate (2.78g, 19.97mmol) and acetonitrile (30mL), placed at 80°C for 12 hours to stop the reaction, the reaction solution was directly concentrated, and the residue was separated by column chromatography (dichloromethane / methanol (v / v)=20 / 1) to obtain the title Compound was a white solid (1.5 g, 59%). MS(ESI,pos.ion)m / z:259.25[M+H] + ;
[0192] 1 H NMR (400MHz, CDCl 3 )δ(ppm)4.45(s,1H),3.79–3.73(m,2H),3.45(dd,J=6.9,5.3Hz,1H),2.95(s,2H),2.61–2.53(m,2H) ,2.05(t,J=9.7Hz,2H),1.92(d,J=11.9Hz,2H),1.68(dt,J=10.8,5.4Hz,2H),1.41(s,9H),1.26(d, J=15.8Hz, 2H).
[0193] Step 2) Synthesis of (1-(3-...
Embodiment 2
[0205] Example 2 Synthesis of 2-(3-(4-amino-5-chloro-2-methoxybenzamido)piperidin-1-yl)propoxy)pyrimidine-4-carboxylic acid
[0206]
[0207] Step 1) of 2-(3-(4-((tert-butoxycarbonyl)amino)piperidin-1-yl)propoxy)pyrimidine-4-carboxylic acid methyl ester synthesis
[0208] Weigh (1-(3-hydroxypropyl) piperidin-4-yl) tert-butyl carbamate (300mg, 1.16mmol) in a 50mL single-necked bottle, add anhydrous tetrahydrofuran (6mL) and sodium hydride (55mg, 1.39mmol ), stirred for 30 minutes, and then a solution of methyl 2-chloropyrimidine-4-carboxylate (260 mg, 1.51 mmol) dissolved in anhydrous tetrahydrofuran (4 mL) was added dropwise to the reaction flask, and stirred at room temperature for 10 hours. The reaction was stopped, the reaction solution was directly concentrated, and the residue was separated by column chromatography (petroleum ether / ethyl acetate (v / v)=1 / 1) to obtain the title compound as a white solid (160 mg, 35%).
[0209] MS(ESI,pos.ion)m / z:395.20[M+H] + ;
...
Embodiment 3
[0223] Example 3 Synthesis of 4-amino-5-chloro-N-(1-(3-(cyclopentyloxy)propyl)piperidin-4-yl)-2-methoxybenzamide
[0224]
[0225] Step 1) Synthesis of (1-(3-(cyclopentyloxy) propyl) piperidin-4-yl) tert-butyl carbamate
[0226] Weigh (1-(3-hydroxypropyl) piperidin-4-yl) tert-butyl carbamate (1.00g, 3.87mmol) in a 100mL single-necked bottle, add dichloromethane (20mL), add silver carbonate (3.2 g, 11.7mmol) and silver perchlorate (250mg, 1.21mmol), add iodocyclopentane (2.37g, 12mmol) dropwise, and react in the dark at room temperature under nitrogen protection for 7 hours. The reaction was terminated, filtered with suction, and the filtrate was concentrated. The residue was separated and purified by column chromatography (dichloromethane / methanol (v / v)=20 / 1) to obtain the title compound as a white solid (989 mg, 78%).
[0227] MS(ESI,pos.ion)m / z:327.35[M+H] + .
[0228] 1 H NMR (400MHz, CDCl 3 )δ(ppm)3.99–3.79(m,1H),3.47(t,J=6.0Hz,2H),3.32(s,2H),3.18(s,2H),2.88–2.64...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com