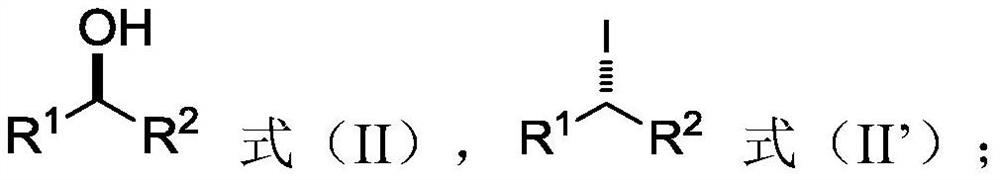Method for deoxygenated functionalization and deoxygenated activation of alcohols and alcohol-functionalized substances
A deoxidation function and activator technology, which is applied in the preparation of amino compounds from amines, organic chemistry, bulk chemical production, etc., can solve the problems that do not conform to the principle of atom economy, the difficulty of separating phosphine oxide by-products, the limitation of the range of nucleophiles, etc. problems, to achieve the effect of wide application range of substrates, easy separation and purification, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0122] The functionalization is amination, the nucleophile is a nitrogen-containing nucleophile, and the method comprises:
[0123] (1) in the first solvent, in the presence of an activator, the alcohol is deoxidized and activated to obtain an iodide intermediate;
[0124] (2) Functionalizing the iodide intermediate in a second solvent in the presence of a nitrogen-containing nucleophile.
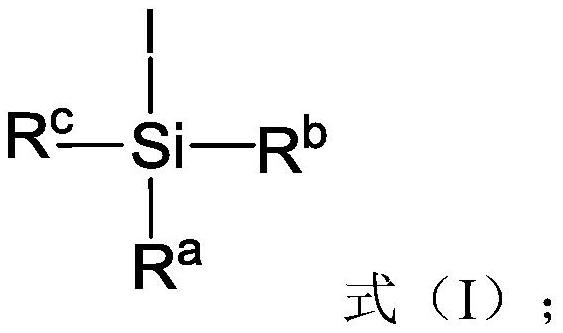
[0125] Preferably, the activator is trimethyliodosilane.
[0126] Preferably, the nitrogen-containing nucleophile is selected from the group consisting of azide salts, amides, sulfonamides, primary aliphatic amines, secondary acyclic or cyclic aliphatic amines, primary aromatic amines, secondary aromatic amines and aza aromatic rings at least one of them.
[0127] Preferably, the amination functionalization is represented by the following formula (2):
[0128]
[0129] Among them, in formula (2), R 1 and R 2 with the aforementioned R 1 and R 2 The definitions correspond to the sam...
specific Embodiment approach
[0132] The nitrogen-containing nucleophile is an azide salt; the second solvent is DMF, and the functionalization conditions include: a temperature of 20-60° C. and a time of 2-16 hours.
[0133] According to another preferred embodiment of the present invention:
[0134] The nitrogen-containing nucleophile is selected from at least one of primary aliphatic amines, secondary (acyclic or cyclic) aliphatic amines, primary aromatic amines, secondary aromatic amines and nitrogen heteroaromatic rings; the second The solvent is acetonitrile, and the functionalization conditions include: heating under reflux, the temperature is 20-100°C, and the time is 0.5-20h.
[0135] According to another preferred embodiment of the present invention:
[0136] The iodide intermediate is a chiral iodide, the nitrogen-containing nucleophile is an azide salt, the amination reaction product is a chiral azide product, and the chiral azide product is the same as the chiral azide product. The optical c...
specific Embodiment approach 2
[0137] The functionalization is sulfurization, the nucleophile is a sulfur-containing nucleophile, and the method includes:
[0138] (1) in the first solvent, in the presence of an activator, the alcohol is deoxidized and activated to obtain an iodide intermediate;
[0139] (2) Functionalizing the iodide intermediate in a second solvent in the presence of a sulfur-containing nucleophile.
[0140] Preferably, the activator is trimethyliodosilane.
[0141] Preferably, the sulfur-containing nucleophile is selected from at least one of aliphatic thiol, substituted or unsubstituted thiophenate, substituted or unsubstituted thiophenol, heteroarylthiol and sodium phenylsulfinate , the optional substituent is selected from at least one of halogen, alkyl, alkoxy, aryl, and amine.
[0142] Preferably, the sulfur functionalization reaction is represented by the following formula (3):
[0143]
[0144] Among them, in formula (3), R 1 and R 2 with the aforementioned R 1 and R 2 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com