Dosage form of TYK2 inhibitor
A dosage form, the technology of BMS-986165, is applied in the field of dosage forms of TYK2 inhibitors, which can solve problems such as unsuccessful efforts, and achieve the effect of improving convenience, improving compliance, and treating inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0088] In a suitable pot add 6-(cyclopropaneamido)-4-((2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl )amino)-N-(methyl-d3)pyridazine-3-carboxamide drug substance and HPMCAS were added to a mixture of acetone and water and mixed to produce a solution. The solution was spray dried under a nitrogen atmosphere (nitrogen provided an inert atmosphere during manufacture). The resulting spray-dried mixture is further dried to provide a spray-dried dispersion (SDD), which can be filled and packaged.
[0089] To manufacture dispersion formulations and dosage forms with extended release characteristics, SDD, lactose anhydrous, microcrystalline cellulose, and HPMCAS were blended together and the blended combinations were screened. The screened composition was blended with magnesium stearate, and the result was subjected to dry granulation (gravity / roller method), followed by milling. This further result was blended with additional magnesium stearate and then tableted to yield a com...
Embodiment B
[0091] The composition of the spray-dried solution used to generate the spray-dried dispersion of solid amorphous BMS-986165 (15% w / w: 85% w / w) molecularly dispersed in a solid HPMCAS-H matrix is shown in Table B below - 1 in.
[0092] Table B-1. Composition of Spray Solution and SDD
[0093]
[0094] Table B-2 below shows the manufacture of amorphous BMS-986165:HPMCAS-H (15%w / w:85%w / w) using a laboratory scale spray dryer with 150-kg / hr drying gas capacity Overview of methods for spray drying dispersions.
[0095] Table B-2. BMS-986165: Manufacturing of HPMCAS-H SDD
[0096]
[0097] While Table B-2 provides that the polymer is added to the solution preparation vessel prior to the addition of the active (BMS-986165), the active (BMS-986165) can be added to the solution preparation vessel prior to the addition of the polymer.
[0098] Table B-3 below lists the solution preparation conditions for 15% BMS-986165:85% HPMCAS-H SDD. The spray drying conditions used to m...
Embodiment C
[0111] Stability of BMS-986165SDD Formulations
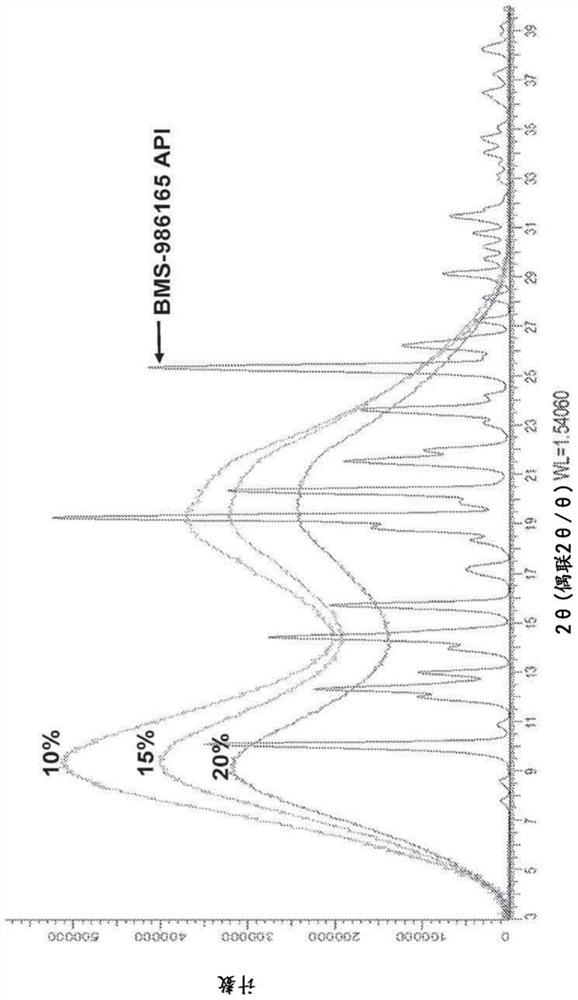
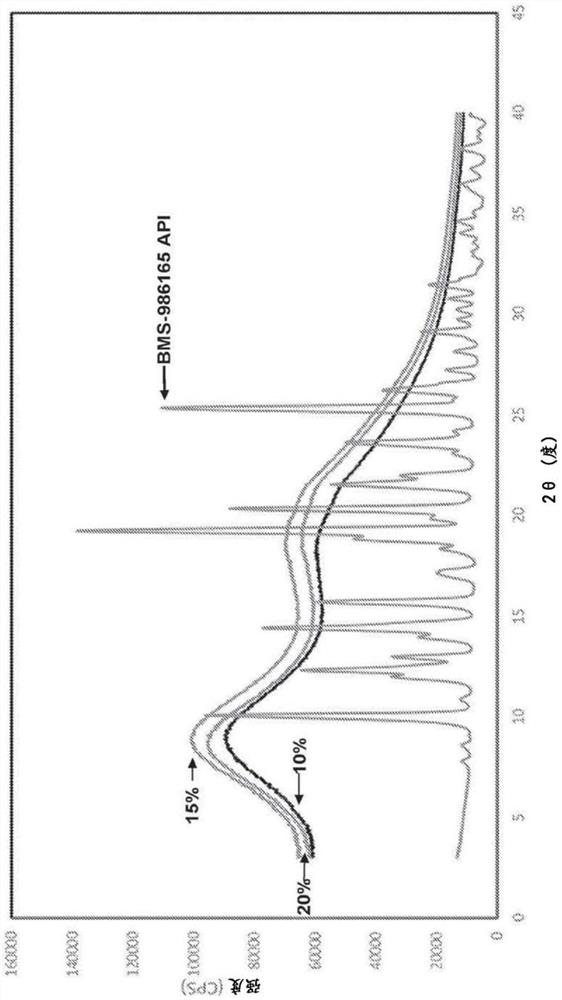
[0112]The physical and chemical stability of a number of 25% w / w BMS-986165SDD with HPMCAS-H was evaluated. HPMCAS SDD is chemically stable under all conditions, but powder X-ray diffraction (PXRD) and modulated differential scanning calorimetry (mDSC) data indicate open storage at 50°C / 75% RH for 1 month and at 40 Crystallization after 3 months of storage in the open at °C / 75% RH. The dissolution performance in the microcentrifugation test was unchanged. There was no evidence of physical instability when the dispersion formulation was stored under 40°C / 75% RH closed or 25°C / 60% RH open conditions for up to 6 months.
[0113] Additional testing was performed to determine API loading levels in HPMCAS-H that would provide chemical and physical stability while still providing the desired dissolution profile. pH shift dissolution testing using the Pion UV probe (pH 2 or pH 6 to pH 6.5) showed that release / maintenance in the gastr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com