Preparation method of indacaterol impurity
A technology of impurity and molar mass, applied in the direction of organic chemistry, etc., to achieve the effects of strong operability, reasonable synthesis process design, and simple and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
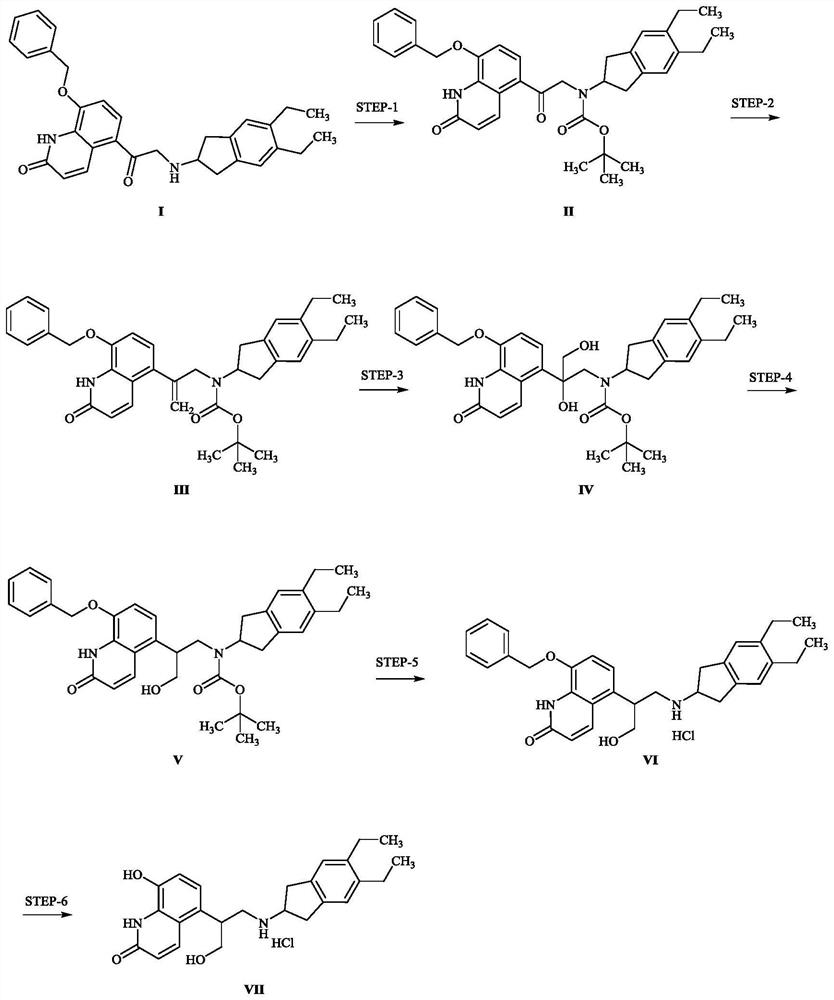
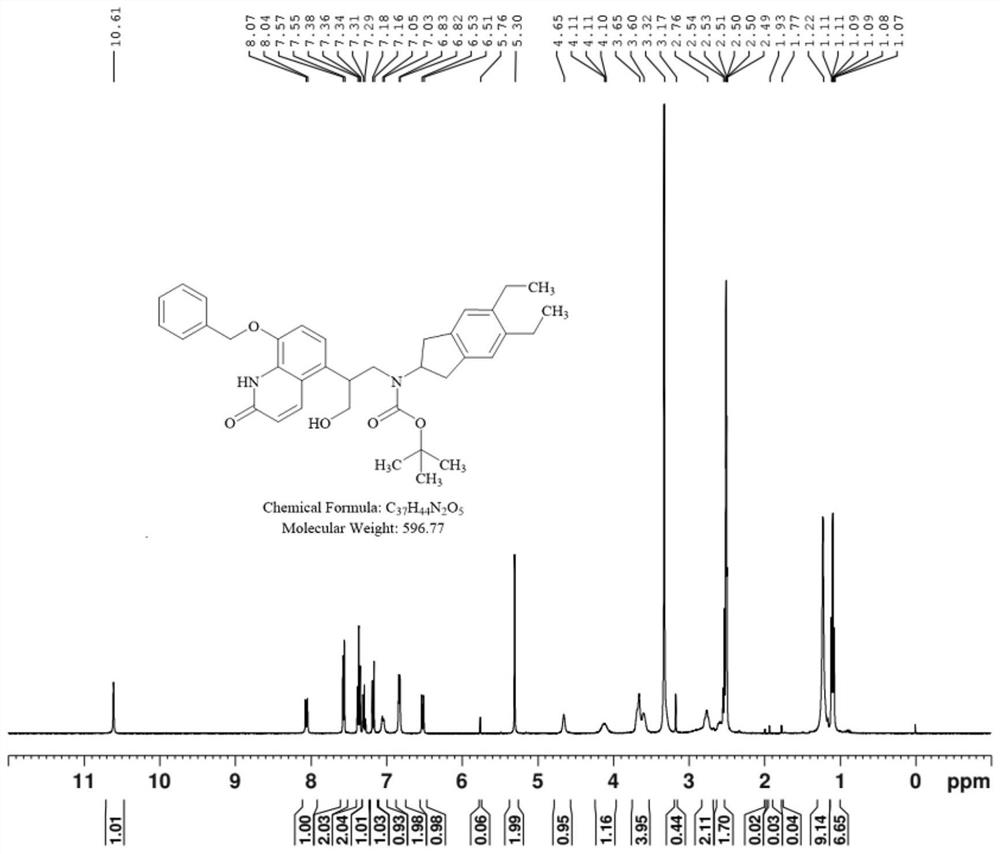
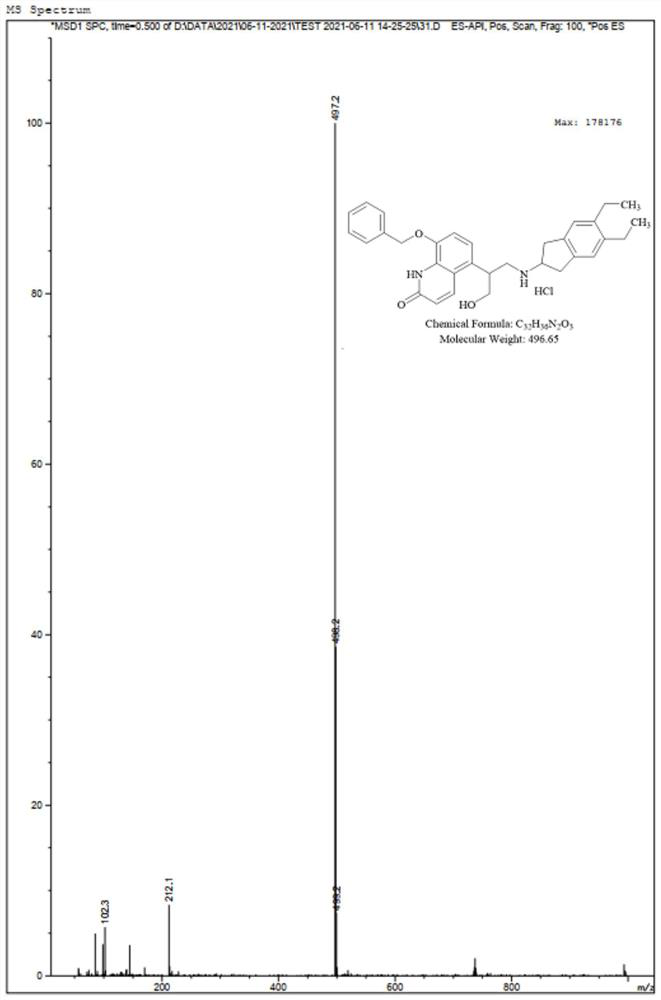
[0033] A kind of preparation method of indacaterol impurity, specifically comprises the following steps, and synthetic route is such as figure 1 shown:
[0034] Preparation of compound II: Compound I (4.00 g) was dissolved in 20 mL of tetrahydrofuran, triethylamine (2.3 mL) and di-tert-butyl dicarbonate (3.62 g) were added, and the mixture was stirred at room temperature for 16 hours. The solvent was evaporated to dryness, and ether was added to stir and the precipitated solid was filtered with suction to obtain 4.70 g of compound II as a white solid with a yield of 97.51%.
[0035] Preparation of compound III: take methyltriphenylphosphine bromide (5.50 g) and suspend in toluene, add potassium tert-butoxide (1.73 g), and stir at room temperature for 1 hour to obtain ylide reagent. Compound II (4.5 g) was dissolved in 45.0 mL of toluene, ylide reagent was added, and the mixture was stirred at room temperature for 3 hours. Add water, extract with ethyl acetate, and purify by ...
Embodiment 2
[0041] A kind of preparation method of indacaterol impurity, specifically comprises the following steps, and synthetic route is such as figure 1 shown:
[0042]Preparation of compound II: take compound I (20.00g) and dissolve it in 100mL of dichloromethane, add N,N-diisopropylethylamine (14.5mL), di-tert-butyl dicarbonate (18.2g), stir at room temperature for 16 Hour. The solvent was evaporated to dryness, diethyl ether was added and the precipitated solid was stirred and filtered with suction to obtain 21.60 g of compound II as a white solid with a yield of 87.45%.
[0043] Preparation of compound III: take methyltriphenylphosphine bromide (13.23g) and suspend it in tetrahydrofuran, add potassium tert-butoxide (6.92g), and stir at room temperature for 1 hour to obtain ylide reagent. Compound II (21.50 g) was dissolved in 215 mL of tetrahydrofuran, ylide reagent was added, and the mixture was stirred at room temperature for 3 hours. Add water, extract with ethyl acetate, an...
Embodiment 3
[0049] A kind of preparation method of indacaterol impurity, specifically comprises the following steps, and synthetic route is such as figure 1 Shown: Preparation of Compound II: Compound I (10.00 g) was dissolved in 50 mL of methanol, triethylamine (2.89 mL) and di-tert-butyl dicarbonate (4.54 g) were added, and the mixture was stirred at room temperature for 8 hours. The solvent was evaporated to dryness, diethyl ether was added and the precipitated solid was stirred and filtered with suction to obtain 9.12 g of compound II as a white solid with a yield of 75.37%.
[0050] Preparation of compound III: take methyltriphenylphosphine bromide (16.61 g) and suspend it in tetrahydrofuran, add sodium hydride (60%, 1.86 g), and stir at room temperature for 1 hour to obtain ylide reagent. Dissolve compound II (9.00 g) in 90.0 mL of tetrahydrofuran, add ylide reagent, and stir at 40 degrees for 5 hours. Add water, extract with ethyl acetate, and purify by column chromatography to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com