Serum-free myogenic differentiation culture medium containing natural compound and application of serum-free myogenic differentiation culture medium
A serum-free culture medium, culture medium technology, applied in animal cells, vertebrate cells, bone/connective tissue cells, etc., can solve the problems of high cost and easy pathogen contamination, so as to alleviate the high cost problem and avoid batch Differences and Effects of Pathogen Contamination Hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Apparent identification of myogenic differentiation state
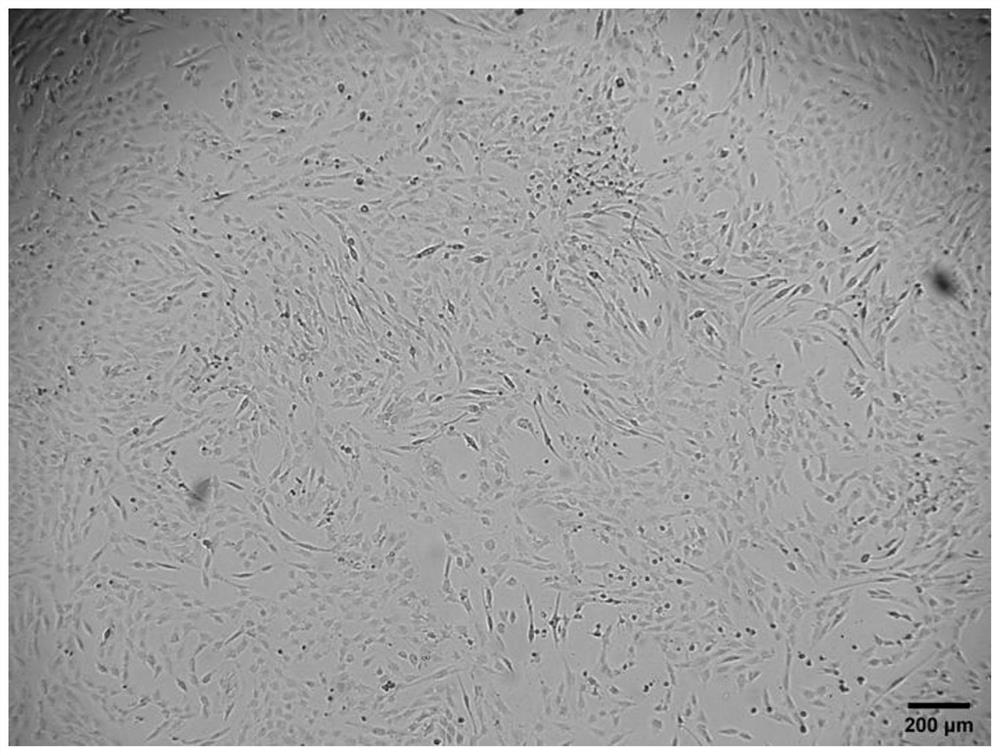
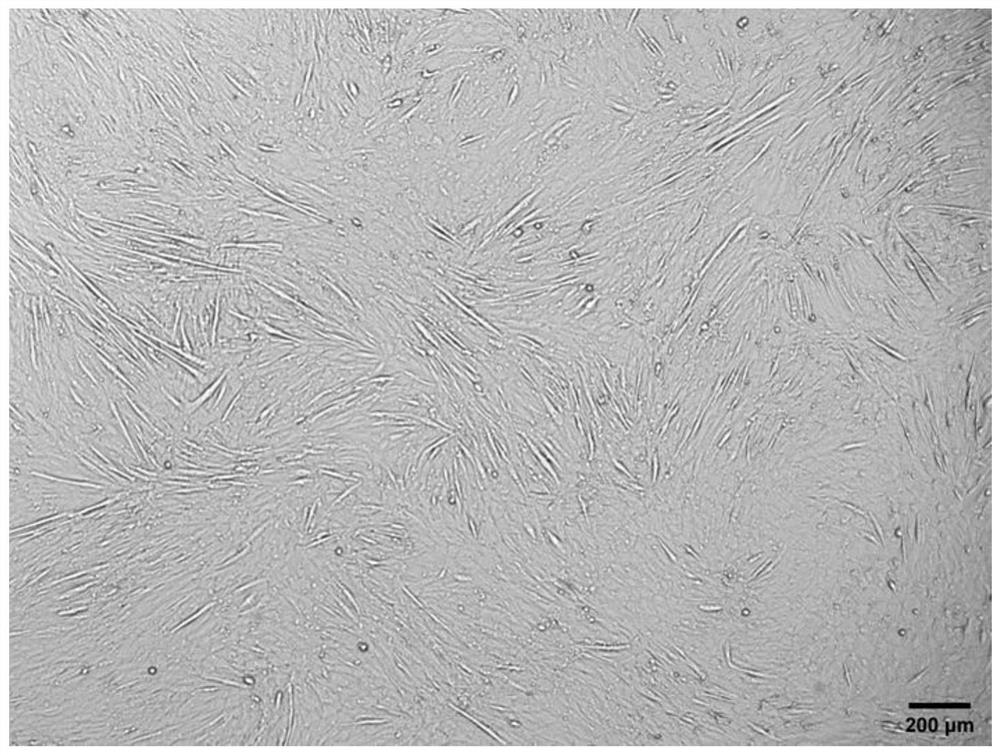
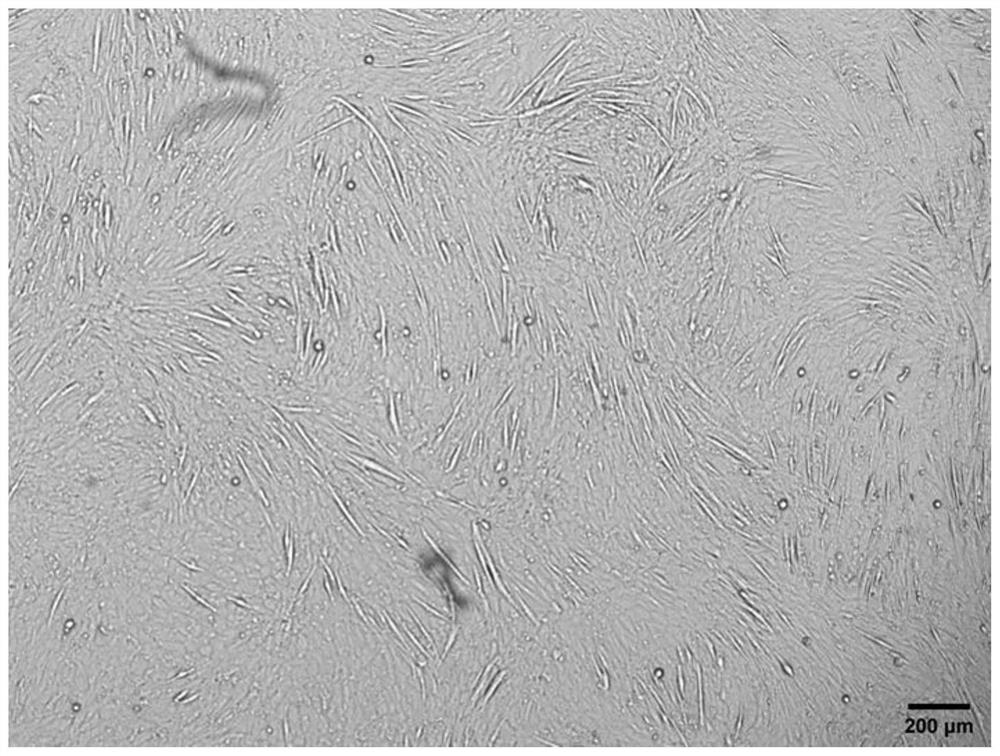
[0052] Press mouse myoblasts C2C12 to 8 × 10 4 Cells / well were inoculated into 24-well plates, a total of 3 wells were inoculated, and cultured in myogenic growth medium for 24 hours. When the confluence of cells reached 70%-90%, the supernatant was aspirated, and the cells in 3 wells were placed in 1 mL Myogenic serum-free differentiation medium, traditional myogenic differentiation medium and modified serum-free myogenic differentiation medium a were differentiated for 5 days, and the corresponding fresh medium was replaced every 2 days, and the differentiation was observed under an inverted microscope5 Apparent changes in astrocytic cells.
[0053] The result is as Figure 1 to Figure 3 As shown, myoblasts undergo significant apoptosis in myogenic serum-free differentiation medium, with only a fraction of cells attached by day 5 of differentiation. However, in the modified serum-free myogenic di...
Embodiment 2
[0054] Example 2 The effect of differentiation-specific protein immunofluorescence characterization of myogenic differentiation
[0055] The three groups of cells differentiated for 5 days in Example 1 were subjected to immunofluorescence detection respectively. The cells were washed three times with PBS, fixed with 300 μL of 4% paraformaldehyde for 15 min, and washed again with PBS three times. After treatment with 300 μL of 0.5% Triton-X100 for 15 min, the cells were washed 3 times with PBS. Add 300 μL of blocking solution (1% BSA, 22.52 mg / mL glycine, 0.1 vol% Tween 20 in PBS) for 30 min, wash with PBS three times, add 250 μL of 1:100 diluted MyHC primary antibody, and incubate at 4°C overnight. After washing three times with PBS, 1:200 secondary antibody was added in the dark, and incubated at 37°C for 1 h. Washed 3 times with PBS and incubated with DAPI for 7 min at room temperature. After washing with PBS three times, the collected images were observed under an inverte...
Embodiment 3
[0057] Example 3 Real-time fluorescence quantitative PCR to detect the expression of myogenic genes and apoptotic genes
[0058] Press mouse myoblasts C2C12 to 8 × 10 4 Cells / well were inoculated into 12-well plates, inoculated into 6 wells, and cultured in myogenic growth medium for 24 hours. When the confluence of cells reached 70%-90%, the supernatant was aspirated, and 1.5 mL of myogenic Serum differentiation medium, traditional myogenic differentiation medium and modified serum-free myogenic differentiation medium a, 2 wells in each group, 1 well differentiated for 3 days, the other well differentiated for 5 days, and the corresponding fresh ones were replaced every 2 days. culture medium. Extracted RNA was reverse transcribed into cDNA on the third or fifth day.
[0059] (1) Harvest cells: discard the medium, wash with PBS, and remove the residual medium. Add 0.25% trypsin for digestion, and after the cells fall off, add twice the volume of trypsin to stop the digesti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com