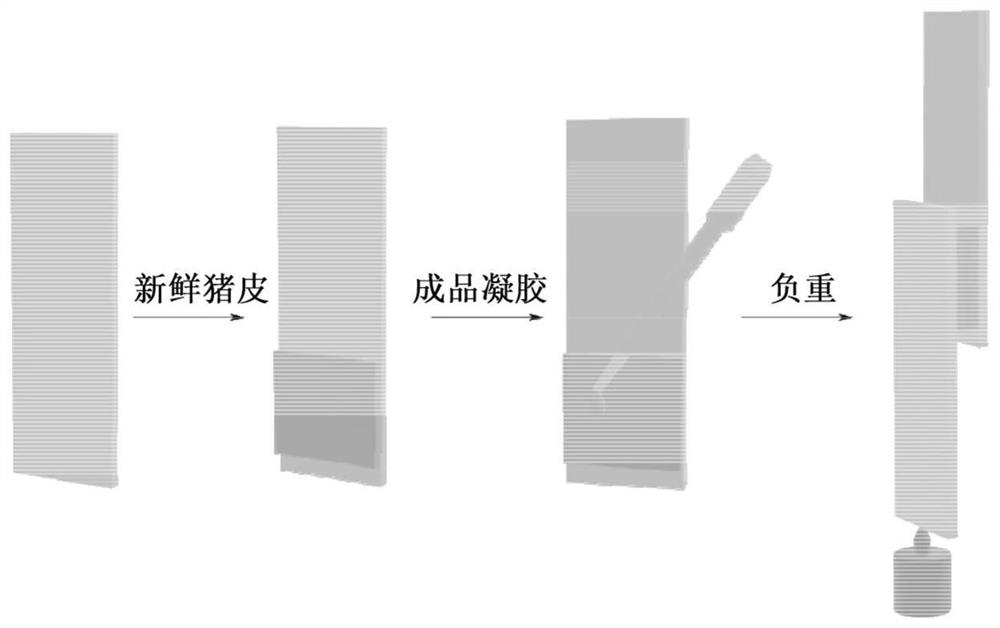
Adhesive sodium hyaluronate gel for injection and preparation method thereof
A technology of sodium hyaluronate and hyaluronic acid, which is applied in prosthesis, medical science, etc., can solve the problems of weak affinity of sodium hyaluronate gel, and achieve the effect of strong binding force, easy injection, and short dialysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] On the one hand, the application provides a kind of preparation method of adhesive sodium hyaluronate gel for injection, it is characterized in that, it comprises the following steps:
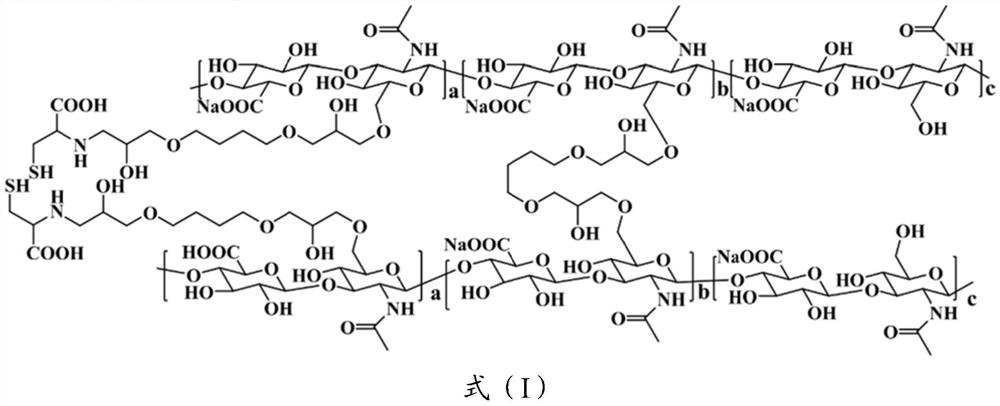
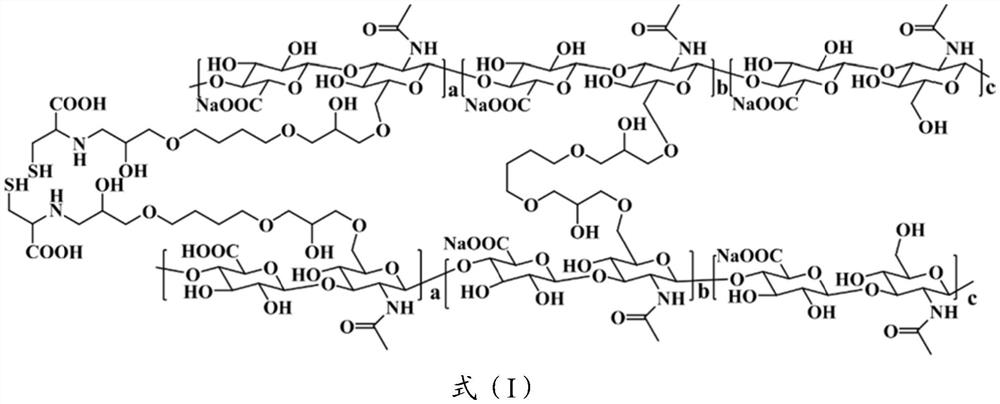
[0036] Synthesize cross-linking agent A: Synthesize cross-linking agent A containing disulfide bonds;
[0037] Cross-linking: The gel L is obtained by reacting sodium hyaluronate with the cross-linking agent A and the cross-linking agent B;
[0038] Preparation of Gel M: Gel M is obtained by treating the Gel L with glutathione;
[0039] Treatment of Gel M: Gel M is dialyzed to obtain the adhesive sodium hyaluronate gel for injection.
[0040] "Glutathione" (GSH) in this application is a tripeptide containing a gamma-amide bond and a sulfhydryl group, consisting of glutamic acid, cysteine and glycine, present in almost every cell of the body. Glutathione helps maintain normal immune system function and has antioxidant, integrated detoxification properties. The sulfhydryl group on cys...
Embodiment 1
[0077] (1) Mix 0.042mol cystine and 0.084mol 1,4-butanediol diglycidyl ether in 100mL, 0.1mol / L NaOH solution, stir and react in a water bath at 20°C for 8 hours, separate and purify to obtain 0.035mol containing Disulfide Epoxy Crosslinker A
[0078]
[0079] (2) Dissolve 939.0 g of sodium hyaluronate (molecular weight 800 kDa, the amount of sodium hyaluronate repeating unit is 2.33 mol) in 100 mL of NaOH solution with a mass fraction of 1.5%, stir and dissolve to obtain sodium hyaluronate solution, the cross-linking agent A and BDDE are mixed and added to the sodium hyaluronate solution, and the cross-linking reaction is carried out at 30 ° C for 8 hours to obtain a cross-linked gel L, wherein the amount of the cross-linking agent A and BDDE is The ratio is 0.5:1, and the ratio of the amount of the total substance of the cross-linking agent A and BDDE to the amount of the substance of the sodium hyaluronate repeating unit is 0.045:1;
[0080] (3) the gel L obtained in st...
Embodiment 2
[0085] (1) Mix 0.042mol cystine and 0.084mol 1,4-butanediol diglycidyl ether in 100mL, 0.1mol / L NaOH solution, stir and react in a water bath at 10°C for 8 hours, separate and purify to obtain 0.035mol containing Disulfide Epoxy Crosslinker A
[0086]
[0087] (2) Dissolve 471.5g sodium hyaluronate (molecular weight 300kDa, the amount of sodium hyaluronate repeating unit is 1.17mol) in 100mL, 1.5% NaOH solution, stir and dissolve to obtain a sodium hyaluronate solution, put The cross-linking agent A and BDDE are mixed and added to the sodium hyaluronate solution, and the cross-linking reaction is carried out at 25 ° C for 8 hours to obtain the cross-linked gel L. The ratio of the amount of the cross-linking agent A to the BDDE is 1:1, the ratio of the total amount of the crosslinking agent A and BDDE to the amount of the sodium hyaluronate repeating unit is 0.06:1;
[0088] (3) the gel L obtained in step (2) is broken by mechanical force, and crossed through a 60-mesh siev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com